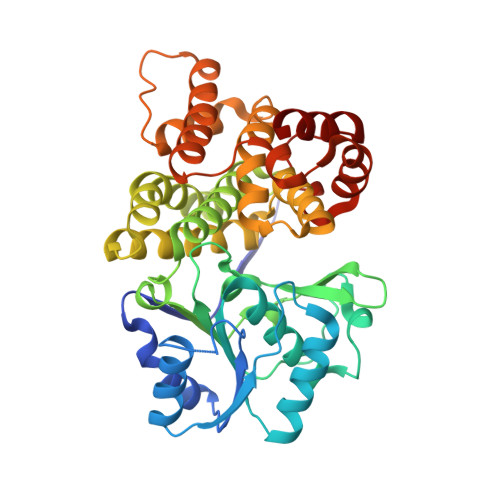
1,3-propanediol dehydrogenase from Klebsiella pneumoniae: decameric quaternary structure and possible subunit cooperativity
Marcal, D., Rego, A.T., Carrondo, M.A., Enguita, F.J.(2009) J Bacteriol 191: 1143-1151
- PubMed: 19011020
- DOI: https://doi.org/10.1128/JB.01077-08
- Primary Citation of Related Structures:
3BFJ - PubMed Abstract:
Klebsiella pneumoniae is a nosocomial pathogen frequently isolated from opportunistic infections, especially in clinical environments. In spite of its potential pathogenicity, this microorganism has several metabolic potentials that could be used in biotechnology applications. K. pneumoniae is able to metabolize glycerol as a sole source of carbon and energy. 1,3-Propanediol dehydrogenase is the core of the metabolic pathway for the use of glycerol. We have determined the crystallographic structure of 1,3-propanediol dehydrogenase, a type III Fe-NAD-dependent alcohol dehydrogenase, at 2.7-A resolution. The structure of the enzyme monomer is closely related to that of other alcohol dehydrogenases. The overall arrangement of the enzyme showed a decameric structure, formed by a pentamer of dimers, which is the catalytic form of the enzyme. Dimers are associated by strong ionic interactions that are responsible for the highly stable in vivo packing of the enzyme. Kinetic properties of the enzyme as determined in the article would suggest that this decameric arrangement is related to the cooperativity between monomers.
Organizational Affiliation:
Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa, 2781-901 Oeiras, Portugal.