Common architecture of the flagellar type III protein export apparatus and F- and V-type ATPases
Ibuki, T., Imada, K., Minamino, T., Kato, T., Miyata, T., Namba, K.(2011) Nat Struct Mol Biol 18: 277-282
- PubMed: 21278755
- DOI: https://doi.org/10.1038/nsmb.1977
- Primary Citation of Related Structures:
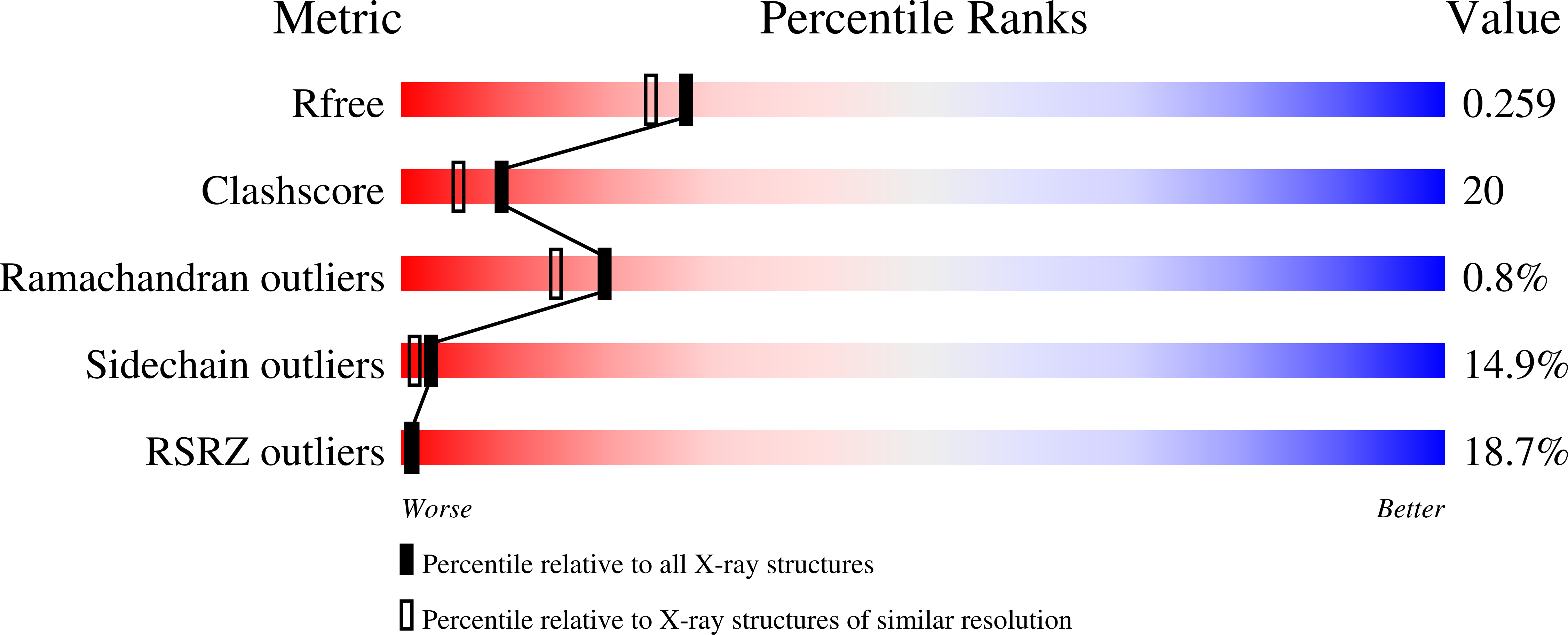
3AJW - PubMed Abstract:
The proteins that form the bacterial flagellum are translocated to its distal end through the central channel of the growing flagellum by the flagellar-specific protein export apparatus, a family of the type III protein secretion system. FliI and FliJ are soluble components of this apparatus. FliI is an ATPase that has extensive structural similarity to the α and β subunits of F(o)F(1)-ATP synthase. FliJ is essential for export, but its function remains obscure. Here we show that the structure of FliJ derived from Salmonella enterica serovar Typhimurium is remarkably similar to that of the two-stranded α-helical coiled-coil part of the γ subunit of F(o)F(1)-ATP synthase and that FliJ promotes the formation of FliI hexamer rings by binding to the center of the ring. These results suggest that the type III protein export system and F- and V-type ATPases share a similar mechanism and an evolutionary relationship.
Organizational Affiliation:
Osaka University, Osaka, Japan.