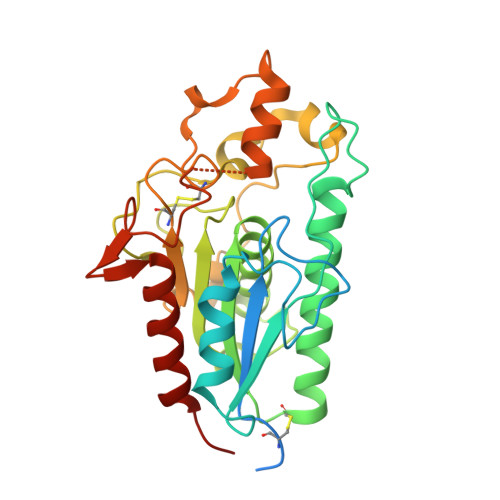
Functional characterisation of a lipase essential for mycobacterial viability
Crellin, P.K., Vivian, J.P., Scoble, J., Chow, F.M., West, N.P., Brammananth, R., Proellocks, N.I., Shahine, A., Le Nours, J., Wilce, M.C.J., Britton, W.J., Coppel, R.L., Rossjohn, J., Beddoe, T.To be published.