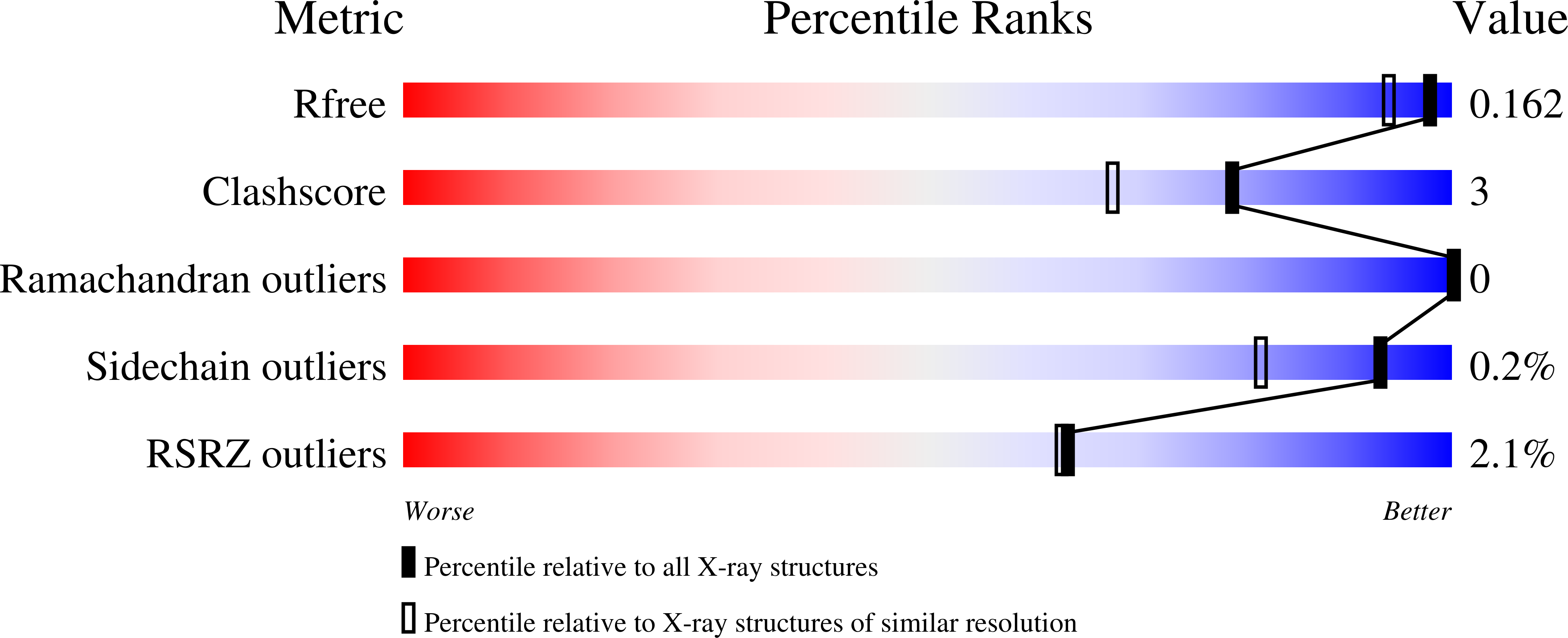
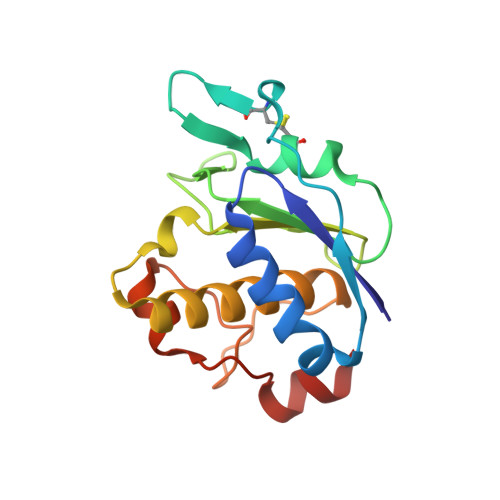
Crystal Structures of Archaemetzincin Reveal a Moldable Substrate-Binding Site.
Graef, C., Schacherl, M., Waltersperger, S., Baumann, U.(2012) PLoS One 7: 43863
- PubMed: 22937112
- DOI: https://doi.org/10.1371/journal.pone.0043863
- Primary Citation of Related Structures:
3ZVS, 4A3W, 4AXQ - PubMed Abstract:
Archaemetzincins are metalloproteases occurring in archaea and some mammalia. They are distinct from all the other metzincins by their extended active site consensus sequence HEXXHXXGXXHCX(4)CXMX(17)CXXC featuring four conserved cysteine residues. Very little is known about their biological importance and structure-function relationships.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of Bern, Bern, Switzerland.