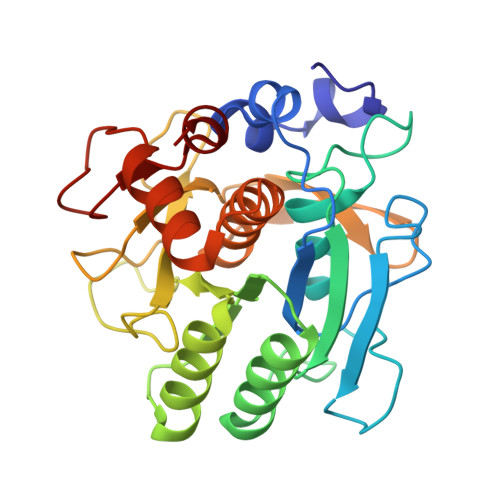
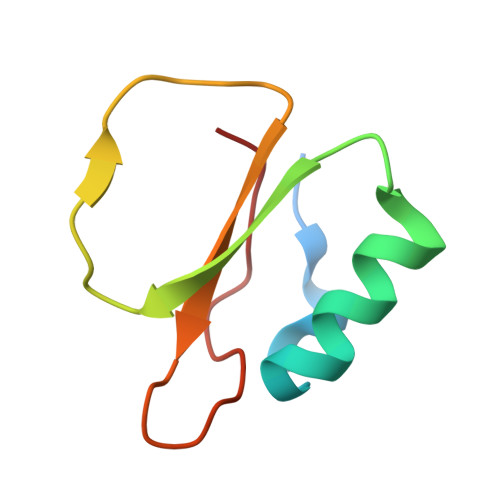
Calcium binding to thermitase. Crystallographic studies of thermitase at 0, 5, and 100 mM calcium.
Gros, P., Kalk, K.H., Hol, W.G.(1991) J Biol Chem 266: 2953-2961
- PubMed: 1993669
- DOI: https://doi.org/10.2210/pdb3tec/pdb
- Primary Citation of Related Structures:
3TEC - PubMed Abstract:
The three-dimensional crystal structure of thermitase complexed with eglin-c in the presence of 100 mM calcium has been determined and refined at 2.0-A resolution to a R-factor of 16.8%. This crystal structure is compared with previously determined structures of thermitase at 0 and 5 mM calcium concentration. In the presence of 100 mM calcium all three calcium binding sites in thermitase are fully occupied. At 100 mM CaCl2 the "weak" calcium binding is occupied by a calcium ion, which is chelated by three protein ligands and four water molecules in a pentagonal bipyramid geometry. Thermitase has, apparently, a monovalent and divalent cation binding position at 2.5-A distance from each other at this site. At low calcium concentrations the monovalent-ion position is occupied by a sodium or potassium ion. The "medium strength" binding site shows in the presence of 100 mM CaCl2 a square antiprism arrangement with eight ligands, of which seven are donated by the protein. At low calcium concentrations we observe a distorted pentagonal bipyramid coordination at this site. The largest difference between these two conformations is observed for ligand Asp-60, which has two conformations with 0.8-A difference in C alpha positions. The "strong" calcium binding site has a pentagonal bipyramid coordination and is fully occupied in all three structures. Structural changes on binding calcium to the weak and "medium strength" calcium binding sites of thermitase are limited to the direct surroundings of these sites. Thermitase resembles in this respect subtilisin BPN' and does not exhibit long-range shifts as have been reported for proteinase K.
Organizational Affiliation:
BIOSON Research Institute, Department of Chemistry, University of Groningen, The Netherlands.