Crystal Structure of the complex of proteinase K with an antimicrobial nonapeptide, at 2.26 A resolution
Singh, A., Sinha, M., Bhushan, A., Kaur, P., Srinivasan, A., Sharma, S., Singh, T.P.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Proteinase K | 279 | Parengyodontium album | Mutation(s): 0 EC: 3.4.21.64 | 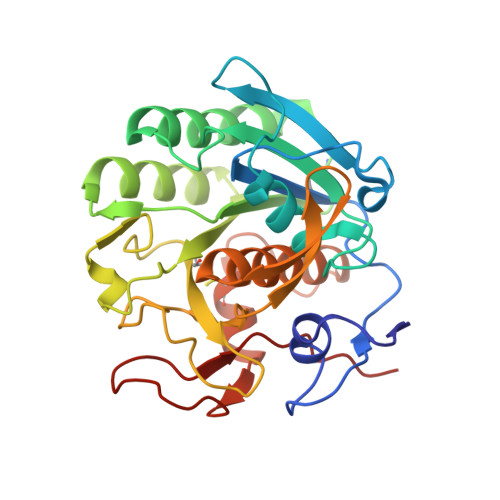 | |
UniProt | |||||
Find proteins for P06873 (Parengyodontium album) Explore P06873 Go to UniProtKB: P06873 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P06873 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 10-mer peptide | 10 | N/A | Mutation(s): 0 | 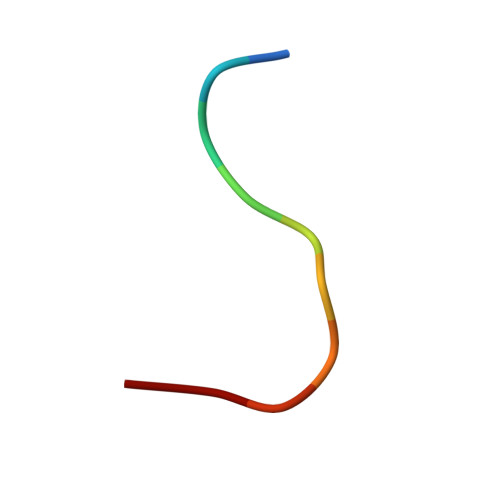 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NO3 Query on NO3 | D [auth A] | NITRATE ION N O3 NHNBFGGVMKEFGY-UHFFFAOYSA-N |  | ||
| CA Query on CA | C [auth A] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 68.29 | α = 90 |
| b = 68.29 | β = 90 |
| c = 108.35 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| AMoRE | phasing |
| CNS | refinement |
| SCALEPACK | data scaling |