Chimeric and Non-Chimeric Foldamer Mimics of the CHR Segment of HIV Protein gp41: Evidence for the Importance of a Large Binding Interface in Six-Helix Bundle Formation
Horne, W.S., Johnson, L.M., Gellman, S.H.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| gp41-5 | 198 | synthetic construct | Mutation(s): 0 | 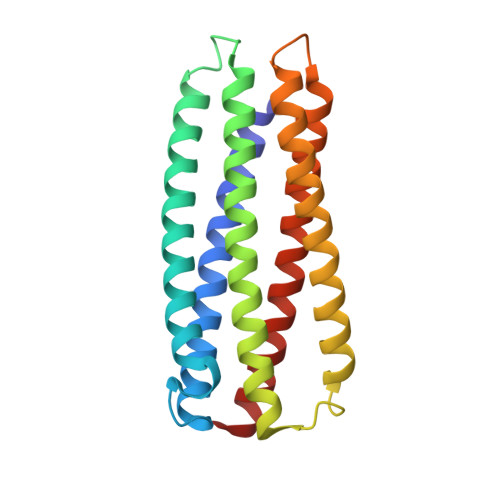 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| alpha/beta-peptide based on HIV gp41 CHR domain sequence | 40 | N/A | Mutation(s): 0 | 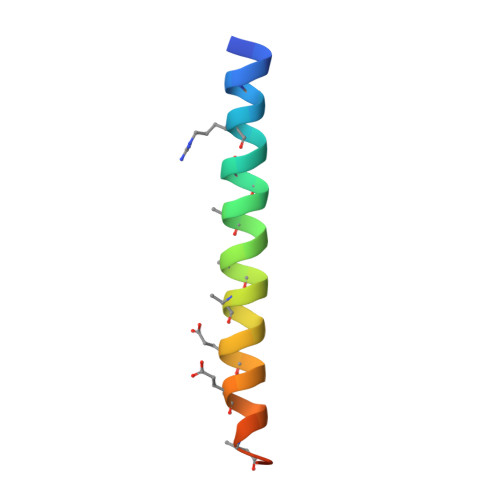 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GOL Query on GOL | C [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| IPA Query on IPA | D [auth A] | ISOPROPYL ALCOHOL C3 H8 O KFZMGEQAYNKOFK-UHFFFAOYSA-N |  | ||
| Modified Residues 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| B3A Query on B3A | B | L-PEPTIDE LINKING | C4 H9 N O2 |  | ALA |
| B3E Query on B3E | B | L-PEPTIDE LINKING | C6 H11 N O4 |  | GLU |
| HMR Query on HMR | B | L-PEPTIDE LINKING | C7 H16 N4 O2 |  | ARG |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 47.407 | α = 90 |
| b = 57.162 | β = 90 |
| c = 102.178 | γ = 90 |
| Software Name | Purpose |
|---|---|
| MD2 | data collection |
| PHASER | phasing |
| REFMAC | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |