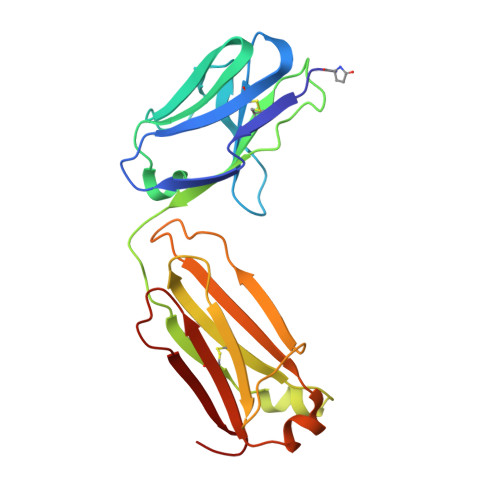
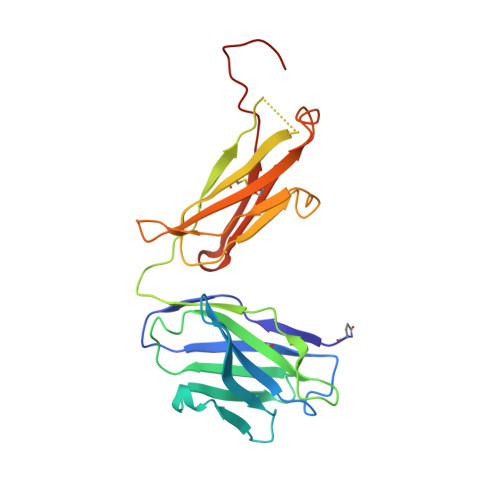
His-tag binding by antibody C706 mimics beta-amyloid recognition.
Teplyakov, A., Obmolova, G., Canziani, G., Zhao, Y., Gutshall, L., Jung, S.S., Gilliland, G.L.(2011) J Mol Recognit 24: 570-575
- PubMed: 20842634
- DOI: https://doi.org/10.1002/jmr.1069
- Primary Citation of Related Structures:
3MCL, 3O11 - PubMed Abstract:
Alzheimer's disease is a progressive neurodegenerative disease characterized by extracellular deposits of β-amyloid (Aβ) plaques. Aggregation of the Aβ(42) peptide leading to plaque formation is believed to play a central role in Alzheimer's disease pathogenesis. Anti-Aβ monoclonal antibodies can reduce amyloid plaques and could possibly be used for immunotherapy. We have developed a monoclonal antibody C706, which recognizes the human Aβ peptide. Here we report the crystal structure of the antibody Fab fragment at 1.7 Å resolution. The structure was determined in two crystal forms, P2(1) and C2. Although the Fab was crystallized in the presence of Aβ(16), no peptide was observed in the crystals. The antigen-binding site is blocked by the hexahistidine tag of another Fab molecule in both crystal forms. The poly-His peptide in an extended conformation occupies a crevice between the light and heavy chains of the variable domain. Two consecutive histidines (His4-His5) stack against tryptophan residues in the central pocket of the antigen-binding surface. In addition, they form hydrogen bonds to the acidic residues at the bottom of the pocket. The mode of his-tag binding by C706 resembles the Aβ recognition by antibodies PFA1 and WO2. All three antibodies recognize the same immunodominant B-cell epitope of Aβ. By similarity, residues Phe-Arg-His of Aβ would be a major portion of the C706 epitope.
Organizational Affiliation:
Centocor R&D, 145 King of Prussia Road, Radnor, PA 19087, USA. ateplyak@its.jnj.com