Iterative structure-based peptide-like inhibitor design against the botulinum neurotoxin serotype A.
Zuniga, J.E., Hammill, J.T., Drory, O., Nuss, J.E., Burnett, J.C., Gussio, R., Wipf, P., Bavari, S., Brunger, A.T.(2010) PLoS One 5: e11378-e11378
- PubMed: 20614028
- DOI: https://doi.org/10.1371/journal.pone.0011378
- Primary Citation of Related Structures:
3NF3 - PubMed Abstract:
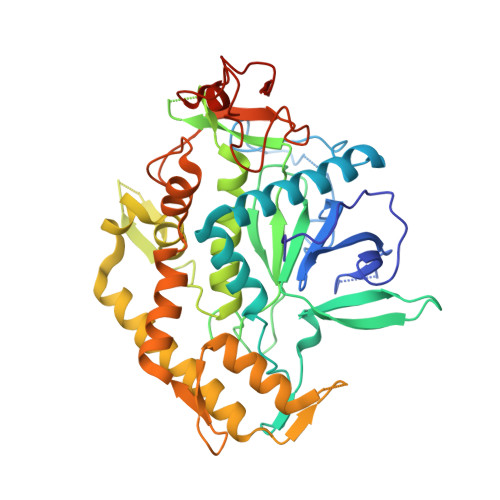
The botulinum neurotoxin serotype A light chain (BoNT/A LC) protease is the catalytic component responsible for the neuroparalysis that is characteristic of the disease state botulism. Three related peptide-like molecules (PLMs) were designed using previous information from co-crystal structures, synthesized, and assayed for in vitro inhibition against BoNT/A LC. Our results indicate these PLMS are competitive inhibitors of the BoNT/A LC protease and their K(i) values are in the nM-range. A co-crystal structure for one of these inhibitors was determined and reveals that the PLM, in accord with the goals of our design strategy, simultaneously involves both ionic interactions via its P1 residue and hydrophobic contacts by means of an aromatic group in the P2' position. The PLM adopts a helical conformation similar to previously determined co-crystal structures of PLMs, although there are also major differences to these other structures such as contacts with specific BoNT/A LC residues. Our structure further demonstrates the remarkable plasticity of the substrate binding cleft of the BoNT/A LC protease and provides a paradigm for iterative structure-based design and development of BoNT/A LC inhibitors.
Organizational Affiliation:
Howard Hughes Medical Institute and Department of Molecular and Cellular Physiology, Stanford University, Stanford, California, United States of America.