crystal structure of the MecA degradation tag
Wang, F., Mei, Z., Qi, Y., Yan, C., Xiang, S., Zhou, Z., Hu, Q., Wang, J., Shi, Y.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Adapter protein mecA 1 | 98 | Bacillus subtilis | Mutation(s): 3 Gene Names: mecA | 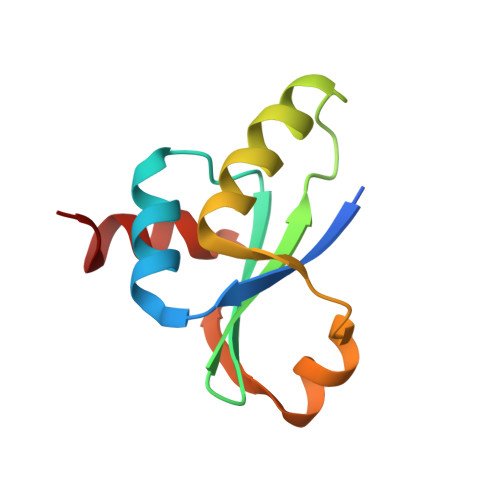 | |
UniProt | |||||
Find proteins for P37958 (Bacillus subtilis (strain 168)) Explore P37958 Go to UniProtKB: P37958 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P37958 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| IOD Query on IOD | AA [auth C] BA [auth C] CA [auth C] DA [auth D] E [auth A] | IODIDE ION I XMBWDFGMSWQBCA-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 69.518 | α = 90 |
| b = 107.27 | β = 90 |
| c = 109.607 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| PHASER | phasing |
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |