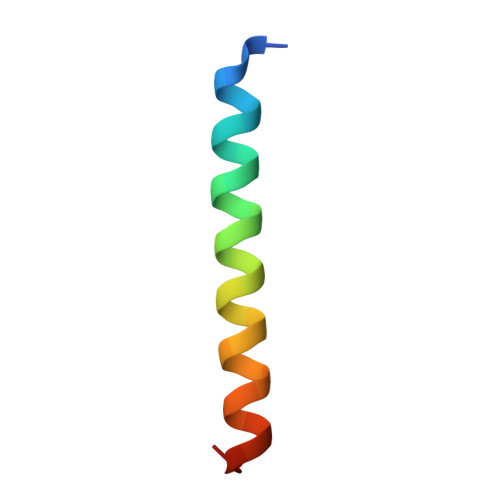
Crystal structure of a super leucine zipper, an extended two-stranded super long coiled coil.
Diao, J.(2010) Protein Sci 19: 319-326
- PubMed: 20027625
- DOI: https://doi.org/10.1002/pro.316
- Primary Citation of Related Structures:
3I1G - PubMed Abstract:
Coiled coil is a ubiquitous structural motif in proteins, with two to seven alpha helices coiled together like the strands of a rope, and coiled coil folding and assembly is not completely understood. A GCN4 leucine zipper mutant with four mutations of K3A, D7A, Y17W, and H18N has been designed, and the crystal structure has been determined at 1.6 A resolution. The peptide monomer shows a helix trunk with short curved N- and C-termini. In the crystal, two monomers cross in 35 degrees and form an X-shaped dimer, and each X-shaped dimer is welded into the next one through sticky hydrophobic ends, thus forming an extended two-stranded, parallel, super long coiled coil rather than a discrete, two-helix coiled coil of the wild-type GCN4 leucine zipper. Leucine residues appear at every seventh position in the super long coiled coil, suggesting that it is an extended super leucine zipper. Compared to the wild-type leucine zipper, the N-terminus of the mutant has a dramatic conformational change and the C-terminus has one more residue Glu 32 determined. The mutant X-shaped dimer has a large crossing angle of 35 degrees instead of 18 degrees in the wild-type dimer. The results show a novel assembly mode and oligomeric state of coiled coil, and demonstrate that mutations may affect folding and assembly of the overall coiled coil. Analysis of the formation mechanism of the super long coiled coil may help understand and design self-assembling protein fibers.
Organizational Affiliation:
Department of Structural Biology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania 15260, USA.