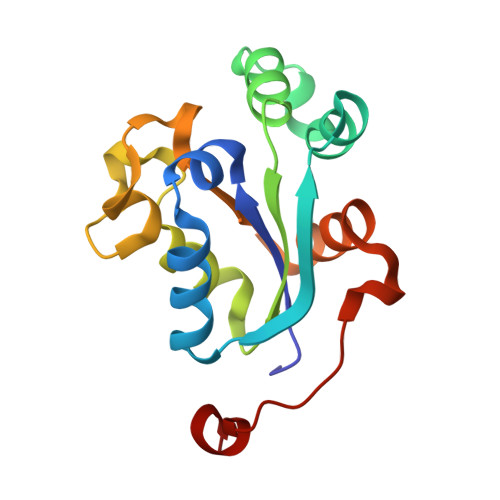
NM23-H2 may play an indirect role in transcriptional activation of c-myc gene expression but does not cleave the nuclease hypersensitive element III1.
Dexheimer, T.S., Carey, S.S., Zuohe, S., Gokhale, V.M., Hu, X., Murata, L.B., Maes, E.M., Weichsel, A., Sun, D., Meuillet, E.J., Montfort, W.R., Hurley, L.H.(2009) Mol Cancer Ther 8: 1363-1377
- PubMed: 19435876
- DOI: https://doi.org/10.1158/1535-7163.MCT-08-1093
- Primary Citation of Related Structures:
3BBB, 3BBC, 3BBF - PubMed Abstract:
The formation of G-quadruplex structures within the nuclease hypersensitive element (NHE) III(1) region of the c-myc promoter and the ability of these structures to repress c-myc transcription have been well established. However, just how these extremely stable DNA secondary structures are transformed to activate c-myc transcription is still unknown. NM23-H2/nucleoside diphosphate kinase B has been recognized as an activator of c-myc transcription via interactions with the NHE III(1) region of the c-myc gene promoter. Through the use of RNA interference, we confirmed the transcriptional regulatory role of NM23-H2. In addition, we find that further purification of NM23-H2 results in loss of the previously identified DNA strand cleavage activity, but retention of its DNA binding activity. NM23-H2 binds to both single-stranded guanine- and cytosine-rich strands of the c-myc NHE III(1) and, to a lesser extent, to a random single-stranded DNA template. However, it does not bind to or cleave the NHE III(1) in duplex form. Significantly, potassium ions and compounds that stabilize the G-quadruplex and i-motif structures have an inhibitory effect on NM23-H2 DNA-binding activity. Mutation of Arg(88) to Ala(88) (R88A) reduced both DNA and nucleotide binding but had minimal effect on the NM23-H2 crystal structure. On the basis of these data and molecular modeling studies, we have proposed a stepwise trapping-out of the NHE III(1) region in a single-stranded form, thus allowing single-stranded transcription factors to bind and activate c-myc transcription. Furthermore, this model provides a rationale for how the stabilization of the G-quadruplex or i-motif structures formed within the c-myc gene promoter region can inhibit NM23-H2 from activating c-myc gene expression.
Organizational Affiliation:
College of Pharmacy,University of Arizona, Tucson, Arizona 85721, USA.