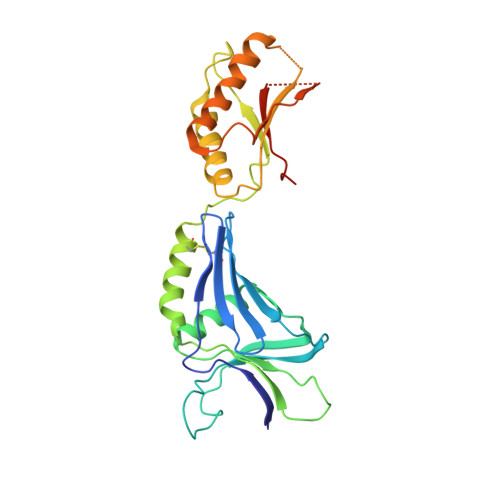
Insights into the stator assembly of the Vibrio flagellar motor from the crystal structure of MotY
Kojima, S., Shinohara, A., Terashima, H., Yakushi, T., Sakuma, M., Homma, M., Namba, K., Imada, K.(2008) Proc Natl Acad Sci U S A 105: 7696-7701
- PubMed: 18505842
- DOI: https://doi.org/10.1073/pnas.0800308105
- Primary Citation of Related Structures:
2ZF8 - PubMed Abstract:
Rotation of the sodium-driven polar flagellum of Vibrio alginolyticus requires four motor proteins: PomA, PomB, MotX, and MotY. PomA and PomB form a sodium-ion channel in the cytoplasmic membrane that functions as a stator complex to couple sodium-ion flux with torque generation. MotX and MotY are components of the T-ring, which is located beneath the P-ring of the polar flagellar basal body and is involved in incorporation of the PomA/PomB complex into the motor. Here, we describe the determination of the crystal structure of MotY at 2.9 A resolution. The structure shows two distinct domains: an N-terminal domain (MotY-N) and a C-terminal domain (MotY-C). MotY-N has a unique structure. MotY-C contains a putative peptidoglycan-binding motif that is remarkably similar to those of peptidoglycan-binding proteins, such as Pal and RmpM, but this region is disordered in MotY. Motility assay of cells producing either of the MotY-N and MotY-C fragments and subsequent biochemical analyses indicate that MotY-N is essential for association of the stator units around the rotor, whereas MotY-C stabilizes the association by binding to the peptidoglycan layer. Based on these observations, we propose a model for the mechanism of stator assembly around the rotor.
Organizational Affiliation:
Division of Biological Science, Graduate School of Science, Nagoya University, Chikusa-Ku, Nagoya 464-8602, Japan.