A Positively Charged Channel within the Smc1/Smc3 Hinge Required for Sister Chromatid Cohesion.
Kurze, A., Michie, K.A., Dixon, S.E., Mishra, A., Itoh, T., Khalid, S., Strmecki, L., Shirahige, K., Haering, C.H., Lowe, J., Nasmyth, K.(2011) EMBO J 30: 364
- PubMed: 21139566
- DOI: https://doi.org/10.1038/emboj.2010.315
- Primary Citation of Related Structures:
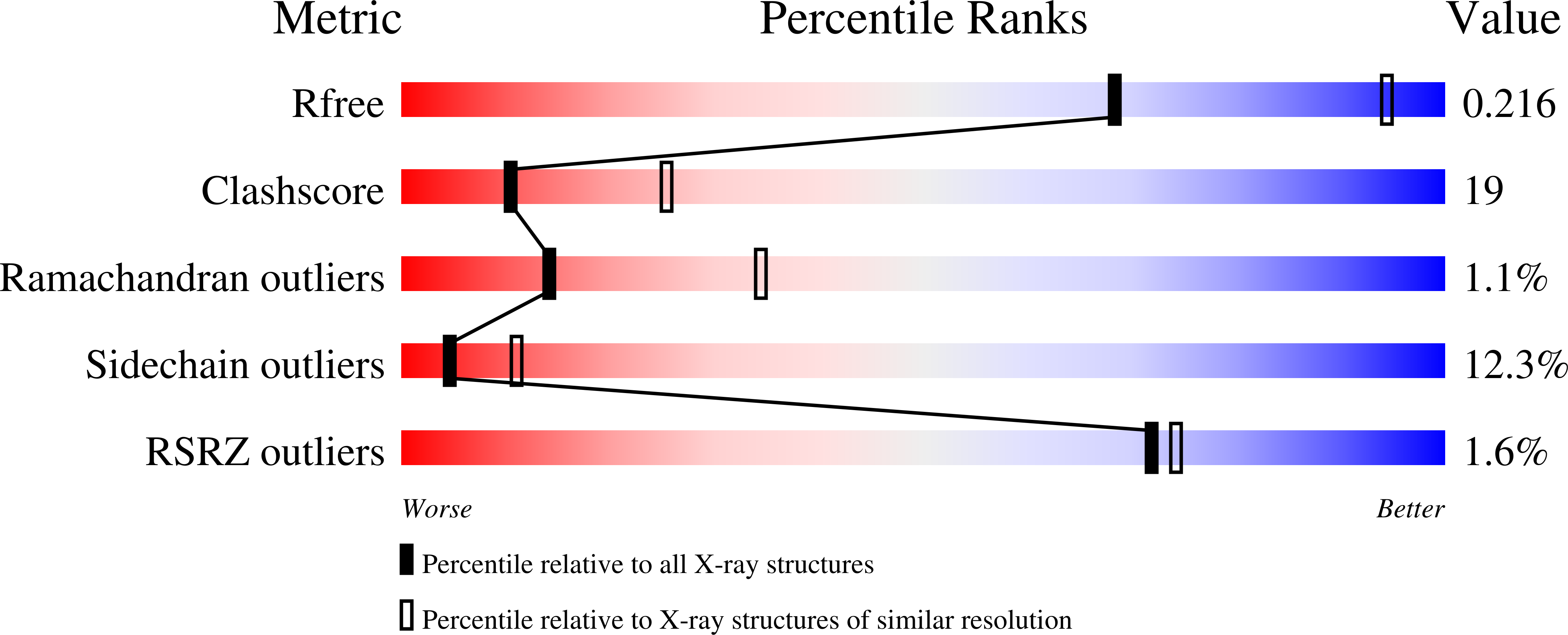
2WD5 - PubMed Abstract:
Cohesin's structural maintenance of chromosome 1 (Smc1) and Smc3 are rod-shaped proteins with 50-nm long intra-molecular coiled-coil arms with a heterodimerization domain at one end and an ABC-like nucleotide-binding domain (NBD) at the other. Heterodimerization creates V-shaped molecules with a hinge at their centre. Inter-connection of NBDs by Scc1 creates a tripartite ring within which, it is proposed, sister DNAs are entrapped. To investigate whether cohesin's hinge functions as a possible DNA entry gate, we solved the crystal structure of the hinge from Mus musculus, which like its bacterial counterpart is characterized by a pseudo symmetric heterodimeric torus containing a small channel that is positively charged. Mutations in yeast Smc1 and Smc3 that together neutralize the channel's charge have little effect on dimerization or association with chromosomes, but are nevertheless lethal. Our finding that neutralization reduces acetylation of Smc3, which normally occurs during replication and is essential for cohesion, suggests that the positively charged channel is involved in a major conformational change during S phase.
Organizational Affiliation:
Department of Biochemistry, University of Oxford, Oxford, UK.