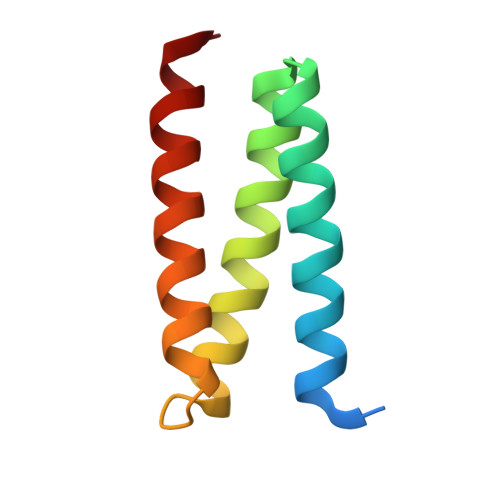
A Role for the Escrt System in Cell Division in Archaea.
Samson, R.Y., Obita, T., Freund, S.M., Williams, R.L., Bell, S.D.(2008) Science 322: 1710
- PubMed: 19008417
- DOI: https://doi.org/10.1126/science.1165322
- Primary Citation of Related Structures:
2W2U - PubMed Abstract:
Archaea are prokaryotic organisms that lack endomembrane structures. However, a number of hyperthermophilic members of the Kingdom Crenarchaea, including members of the Sulfolobus genus, encode homologs of the eukaryotic endosomal sorting system components Vps4 and ESCRT-III (endosomal sorting complex required for transport-III). We found that Sulfolobus ESCRT-III and Vps4 homologs underwent regulation of their expression during the cell cycle. The proteins interacted and we established the structural basis of this interaction. Furthermore, these proteins specifically localized to the mid-cell during cell division. Overexpression of a catalytically inactive mutant Vps4 in Sulfolobus resulted in the accumulation of enlarged cells, indicative of failed cell division. Thus, the archaeal ESCRT system plays a key role in cell division.
Organizational Affiliation:
Medical Research Council (MRC) Cancer Cell Unit, Hills Road, Cambridge CB2 0XZ, UK.