Recognition and Repair of Uv Lesions in Loop Structures of Duplex DNA by Dash-Type Cryptochrome.
Pokorny, R., Klar, T., Hennecke, U., Carell, T., Batschauer, A., Essen, L.-O.(2008) Proc Natl Acad Sci U S A 105: 21023
- PubMed: 19074258
- DOI: https://doi.org/10.1073/pnas.0805830106
- Primary Citation of Related Structures:
2VTB - PubMed Abstract:
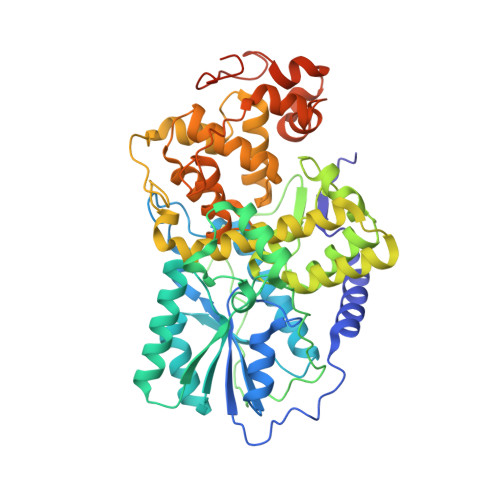
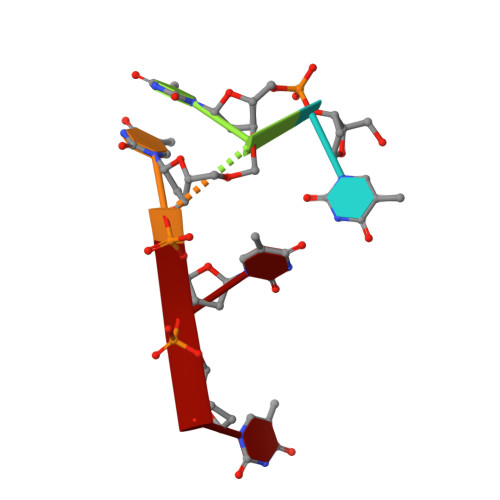
DNA photolyases and cryptochromes (cry) form a family of flavoproteins that use light energy in the blue/UV-A region for the repair of UV-induced DNA lesions or for signaling, respectively. Very recently, it was shown that members of the DASH cryptochrome subclade repair specifically cyclobutane pyrimidine dimers (CPDs) in UV-damaged single-stranded DNA. Here, we report the crystal structure of Arabidopsis cryptochrome 3 with an in-situ-repaired CPD substrate in single-stranded DNA. The structure shows a binding mode similar to that of conventional DNA photolyases. Furthermore, CPD lesions in double-stranded DNA are bound and repaired with similar efficiency as in single-stranded DNA if the CPD lesion is present in a loop structure. Together, these data reveal that DASH cryptochromes catalyze light-driven DNA repair like conventional photolyases but lack an efficient flipping mechanism for interaction with CPD lesions within duplex DNA.
Organizational Affiliation:
Department of Biology, Philipps University, Karl-von-Frisch-Strasse 8, D-35032 Marburg, Germany.