The Crystal Structure of Beta-Alanine Synthase from Drosophila Melanogaster Reveals a Homooctameric Helical Turn-Like Assembly.
Lundgren, S., Lohkamp, B., Andersen, B., Piskur, J., Dobritzsch, D.(2008) J Mol Biol 377: 1544
- PubMed: 18336837
- DOI: https://doi.org/10.1016/j.jmb.2008.02.011
- Primary Citation of Related Structures:
2VHH, 2VHI - PubMed Abstract:
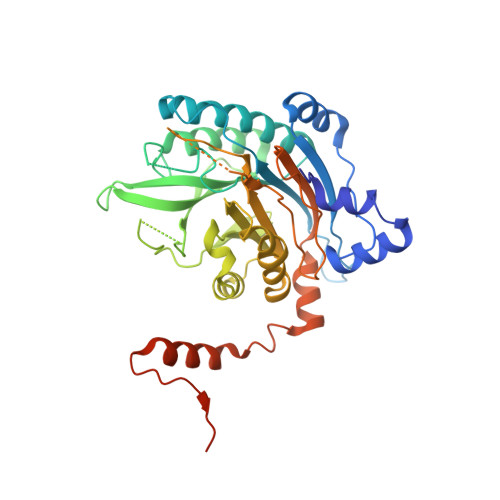
Beta-alanine synthase (betaAS) is the third enzyme in the reductive pyrimidine catabolic pathway, which is responsible for the breakdown of the nucleotide bases uracil and thymine in higher organisms. It catalyzes the hydrolysis of N-carbamyl-beta-alanine and N-carbamyl-beta-aminoisobutyrate to the corresponding beta-amino acids. betaASs are grouped into two phylogenetically unrelated subfamilies, a general eukaryote one and a fungal one. To reveal the molecular architecture and understand the catalytic mechanism of the general eukaryote betaAS subfamily, we determined the crystal structure of Drosophila melanogaster betaAS to 2.8 A resolution. It shows a homooctameric assembly of the enzyme in the shape of a left-handed helical turn, in which tightly packed dimeric units are related by 2-fold symmetry. Such an assembly would allow formation of higher oligomers by attachment of additional dimers on both ends. The subunit has a nitrilase-like fold and consists of a central beta-sandwich with a layer of alpha-helices packed against both sides. However, the core fold of the nitrilase superfamily enzymes is extended in D. melanogaster betaAS by addition of several secondary structure elements at the N-terminus. The active site can be accessed from the solvent by a narrow channel and contains the triad of catalytic residues (Cys, Glu, and Lys) conserved in nitrilase-like enzymes.
Organizational Affiliation:
Department of Medical Biochemistry and Biophysics, Karolinska Institutet, SE-17177 Stockholm, Sweden.