Assembly and Channel Opening in a Bacterial Drug Efflux Machine.
Bavro, V.N., Pietras, Z., Furnham, N., Perez-Cano, L., Fernandez-Recio, J., Pei, X.Y., Truer, R., Misra, R., Luisi, B.(2008) Mol Cell 30: 114
- PubMed: 18406332
- DOI: https://doi.org/10.1016/j.molcel.2008.02.015
- Primary Citation of Related Structures:
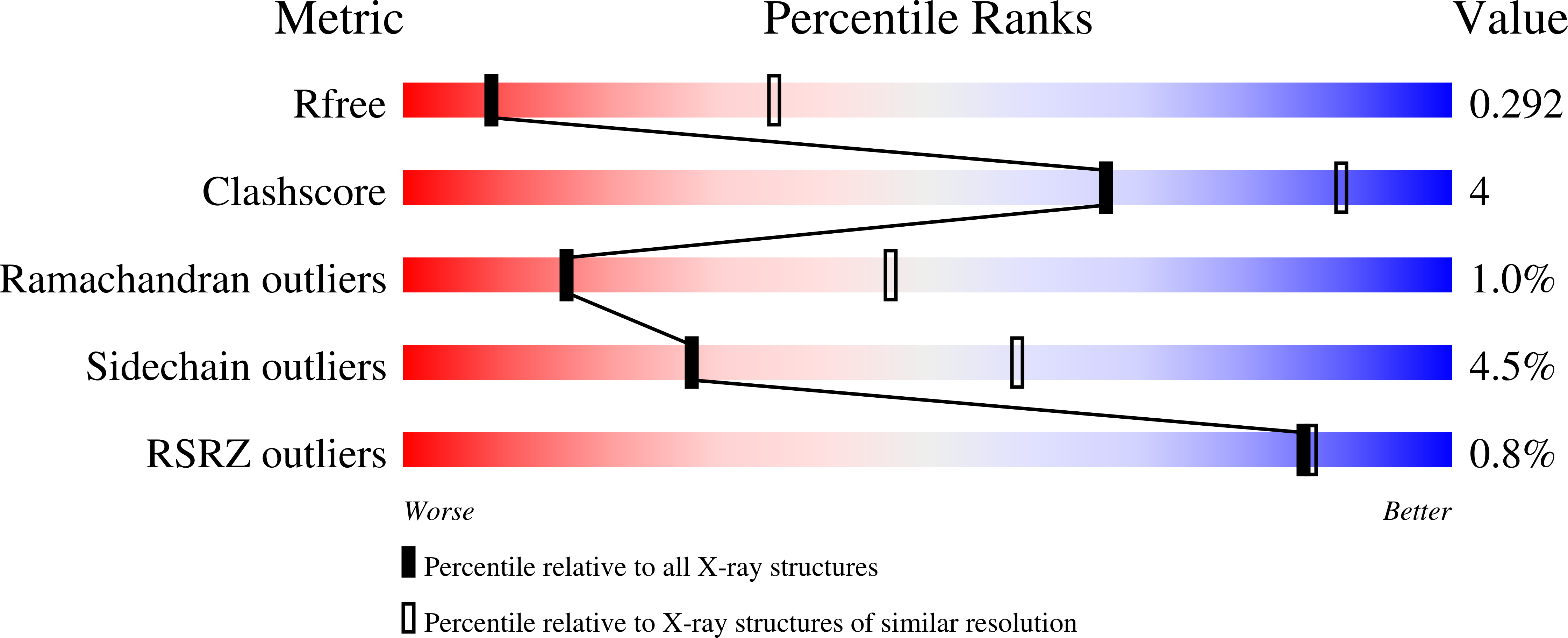
2VDD, 2VDE - PubMed Abstract:
Drugs and certain proteins are transported across the membranes of Gram-negative bacteria by energy-activated pumps. The outer membrane component of these pumps is a channel that opens from a sealed resting state during the transport process. We describe two crystal structures of the Escherichia coli outer membrane protein TolC in its partially open state. Opening is accompanied by the exposure of three shallow intraprotomer grooves in the TolC trimer, where our mutagenesis data identify a contact point with the periplasmic component of a drug efflux pump, AcrA. We suggest that the assembly of multidrug efflux pumps is accompanied by induced fit of TolC driven mainly by accommodation of the periplasmic component.
Organizational Affiliation:
Department of Biochemistry, University of Cambridge, 80 Tennis Court Road, Cambridge CB2 1GA, UK.