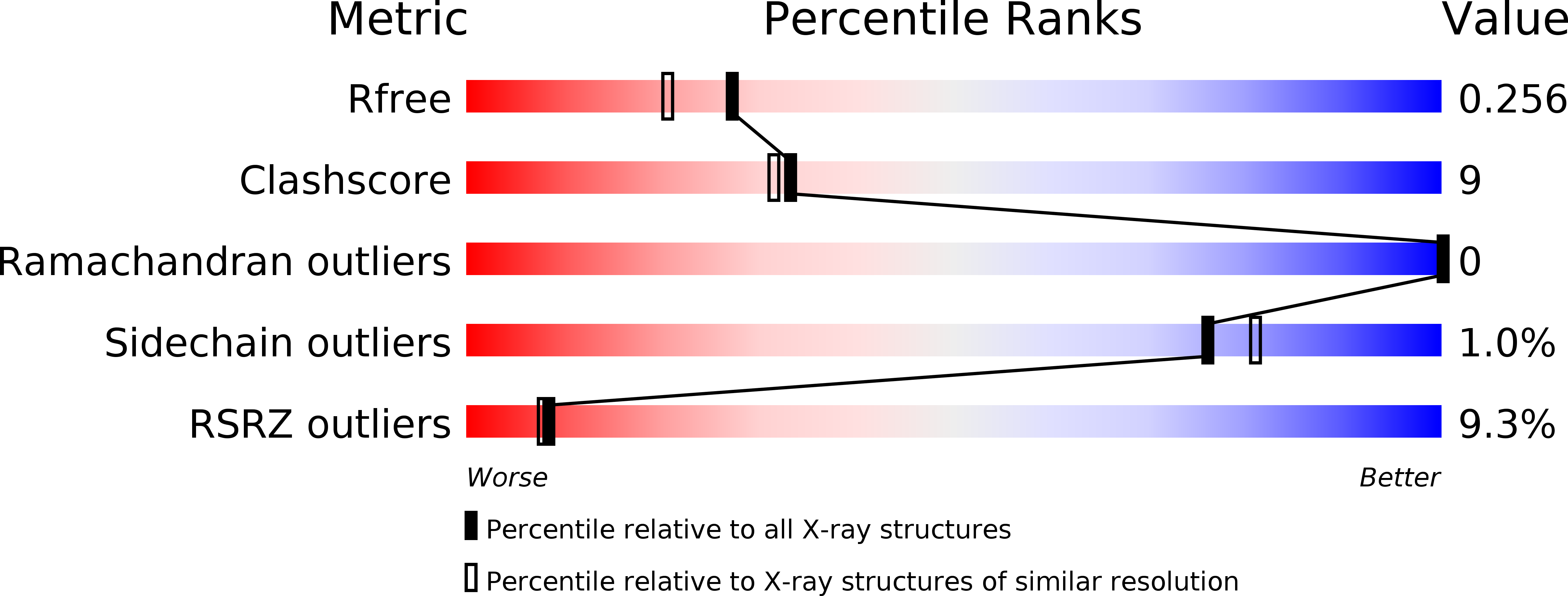
Structure of the Type III Secretion Recognition Protein Yscu from Yersinia Enterocolitica
Wiesand, U., Sorg, I., Amstutz, M., Wagner, S., Van Den Heuvel, J., Luehrs, T., Cornelis, G.R., Heinz, D.W.(2009) J Mol Biol 385: 854
- PubMed: 18976663
- DOI: https://doi.org/10.1016/j.jmb.2008.10.034
- Primary Citation of Related Structures:
2V5G, 2W0R - PubMed Abstract:
The inner-membrane protein YscU has an important role during the assembly of the Yersinia enterocolitica type III secretion injectisome. Its cytoplasmic domain (YscU(C)) recognizes translocators as individual substrates in the export hierarchy. Activation of YscU entails autocleavage at a conserved NPTH motif. Modification of this motif markedly changes the properties of YscU, including translocator export cessation and production of longer injectisome needles. We determined the crystal structures of the uncleaved variants N263A and N263D of YscU(C) at 2.05 A and 1.55 A resolution, respectively. The globular domain is found to consist of a central, mixed beta-sheet surrounded by alpha-helices. The NPTH motif forms a type II beta-turn connecting two beta-strands. NMR analysis of cleaved and uncleaved YscU(C) indicates that the global structure of the protein is retained in cleaved YscU(C). The structure of YscU(C) variant N263D reveals that wild type YscU(C) is poised for cleavage due to an optimal reaction geometry for nucleophilic attack of the scissile bond by the side chain of Asn263. In vivo analysis of N263Q and H266A/R314A YscU variants showed a phenotype that combines the absence of translocator secretion with normal needle-length control. Comparing the structure of YscU to those of related proteins reveals that the linker domain between the N-terminal transmembrane domain and the autocleavage domain can switch from an extended to a largely alpha-helical conformation, allowing for optimal positioning of the autocleavage domain during injectisome assembly.
Organizational Affiliation:
Division of Structural Biology, Helmholtz Centre for Infection Research (HZI), Inhoffenstrasse 7, 38124 Braunschweig, Germany.