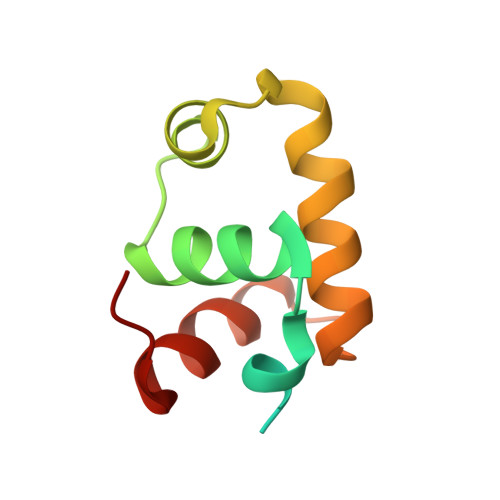
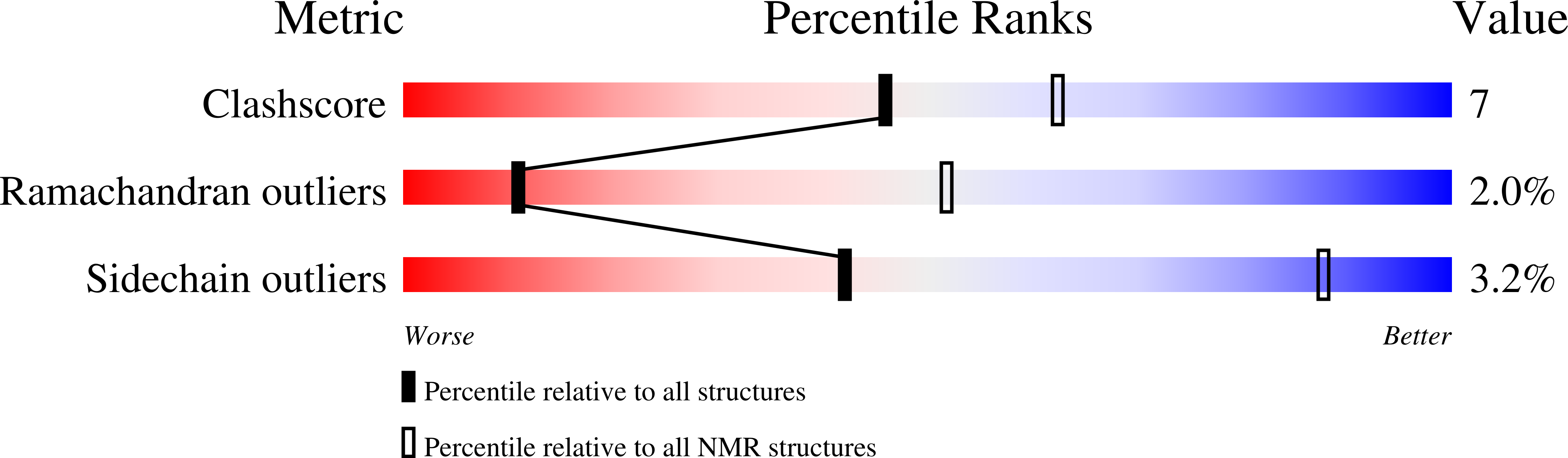The NMR Structure of the Staphylococcus aureus Response Regulator VraR DNA Binding Domain Reveals a Dynamic Relationship between It and Its Associated Receiver Domain
Donaldson, L.W.(2008) Biochemistry 47: 3379-3388
- PubMed: 18293926
- DOI: https://doi.org/10.1021/bi701844q
- Primary Citation of Related Structures:
2RNJ - PubMed Abstract:
In Staphylococcus aureus, a two-component signaling system consisting of the histidine kinase VraS and the response regulator VraR stimulates gene expression in response to antibiotics that inhibit cell wall formation. With respect to understanding the mechanism of the VraSR response and precise interaction of VraR at promoter sites, the structure of the VraR DNA binding domain (DBD) was determined using NMR methods. The DBD demonstrates a four-helix configuration that is shared with the NarL/FixJ family of response regulators and is monomeric in solution. Unobservable amide resonances in VraR NMR spectra coincided with a set of DNA backbone contact sites predicted from a model of a VraR-DNA complex. This observation suggests that a degree of conformational sampling is required to achieve a high-affinity interaction with DNA. On the basis of chemical shift differences and line broadening, an amino-terminal 3 10 helix and a portion of helix H4 identify a continuous surface that may link the DBD to the receiver domain. The full-length VraR protein thermally denatured with a single transition, suggesting that the receiver domain and DBD were integrated and not simply tethered. Of note, the DBD alone denatured at a temperature that was 21 degrees C higher than that of the full-length protein. Thus, the DBD appears to be thermodynamically and structurally sensitive to state of the receiver domain.
Organizational Affiliation:
Department of Biology, York University, 4700 Keele Street, Toronto, Ontario M3J 1P3, Canada. logand@yorku.ca