Structural basis of vta1 function in the multivesicular body sorting pathway.
Xiao, J., Xia, H., Zhou, J., Azmi, I.F., Davies, B.A., Katzmann, D.J., Xu, Z.(2008) Dev Cell 14: 37-49
- PubMed: 18194651
- DOI: https://doi.org/10.1016/j.devcel.2007.10.013
- Primary Citation of Related Structures:
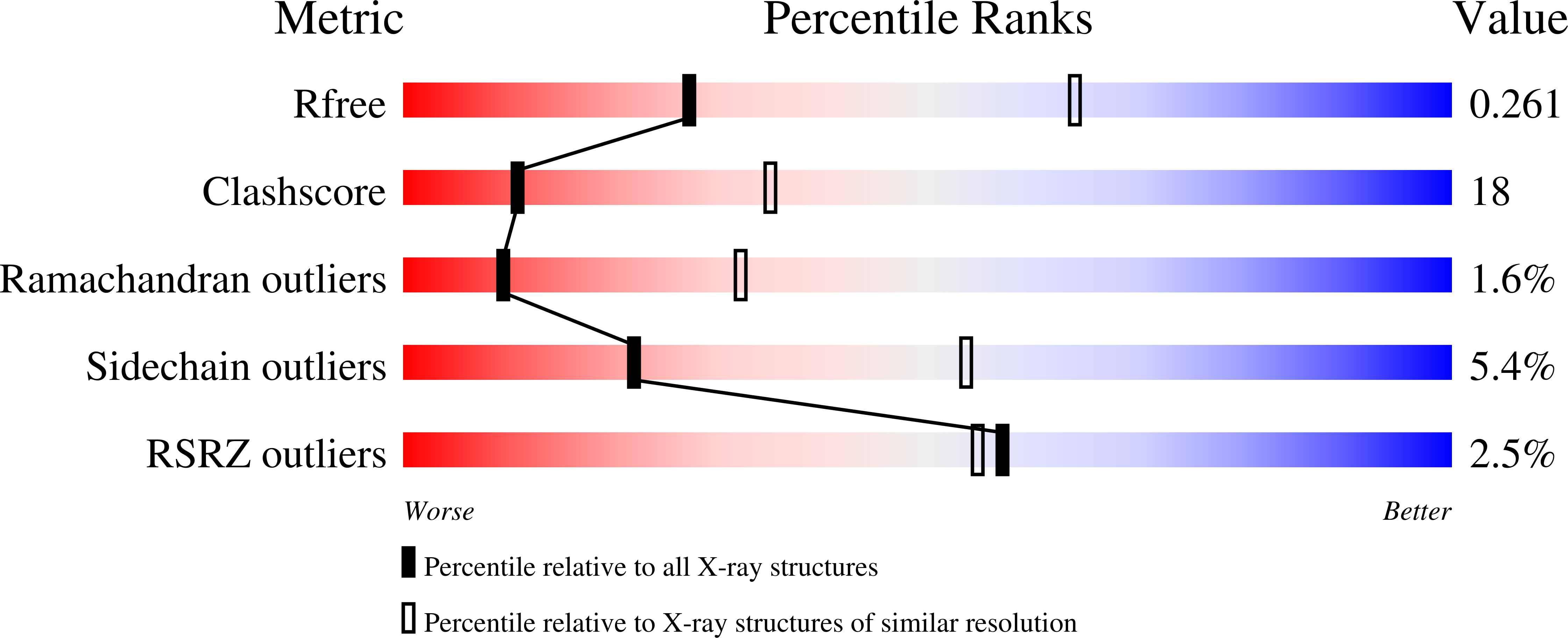
2RKK, 2RKL - PubMed Abstract:
The MVB pathway plays essential roles in several eukaryotic cellular processes. Proper function of the MVB pathway requires reversible membrane association of the ESCRTs, a process catalyzed by Vps4 ATPase. Vta1 regulates the Vps4 activity, but its mechanism of action was poorly understood. We report the high-resolution crystal structures of the Did2- and Vps60-binding N-terminal domain and the Vps4-binding C-terminal domain of S. cerevisiae Vta1. The C-terminal domain also mediates Vta1 dimerization and both subunits are required for its function as a Vps4 regulator. Emerging from our analysis is a mechanism of regulation by Vta1 in which the C-terminal domain stabilizes the ATP-dependent double ring assembly of Vps4. In addition, the MIT motif-containing N-terminal domain, projected by a long disordered linker, allows contact between the Vps4 disassembly machinery and the accessory ESCRT-III proteins. This provides an additional level of regulation and coordination for ESCRT-III assembly and disassembly.
Organizational Affiliation:
Life Sciences Institute and Department of Biological Chemistry, Medical School, University of Michigan, Ann Arbor, MI 48109, USA.