Structure of alpha-glycerophosphate oxidase from Streptococcus sp.: a template for the mitochondrial alpha-glycerophosphate dehydrogenase.
Colussi, T., Parsonage, D., Boles, W., Matsuoka, T., Mallett, T.C., Karplus, P.A., Claiborne, A.(2008) Biochemistry 47: 965-977
- PubMed: 18154320
- DOI: https://doi.org/10.1021/bi701685u
- Primary Citation of Related Structures:
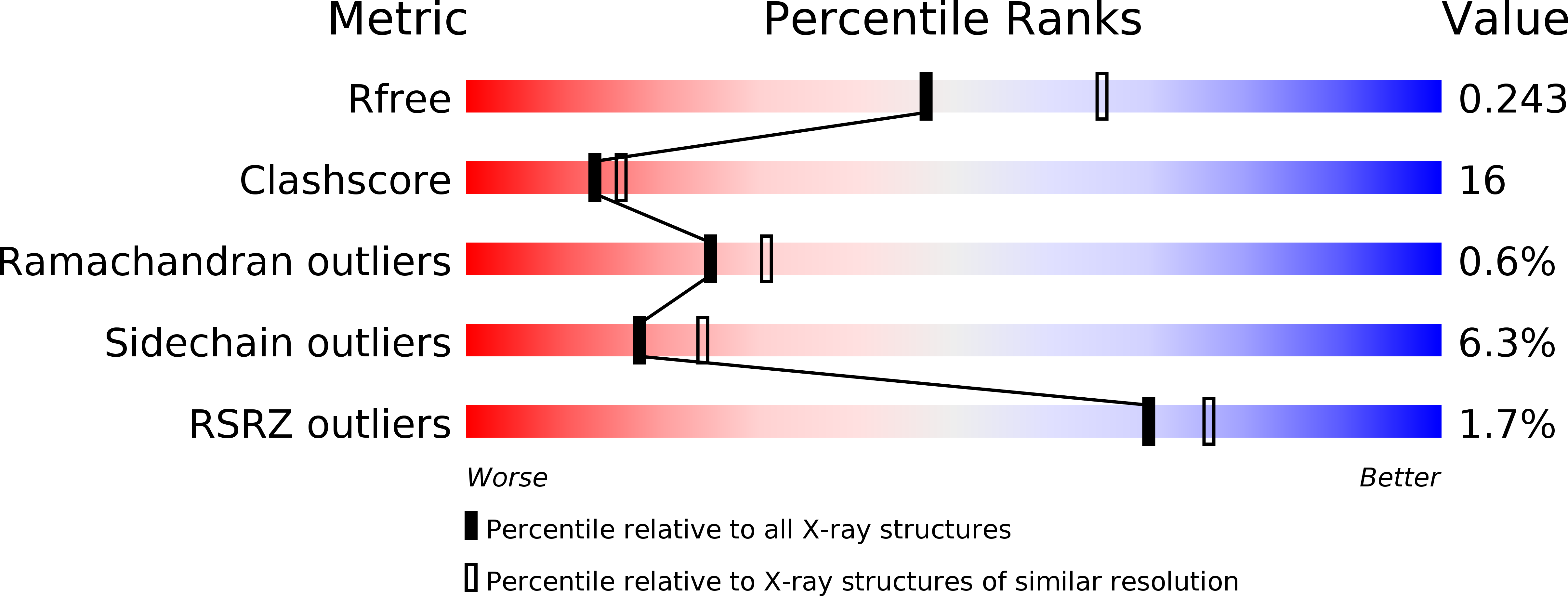
2RGH, 2RGO - PubMed Abstract:
The FAD-dependent alpha-glycerophosphate oxidase (GlpO) from Enterococcus casseliflavus and Streptococcus sp. was originally studied as a soluble flavoprotein oxidase; surprisingly, the GlpO sequence is 30-43% identical to those of the alpha-glycerophosphate dehydrogenases (GlpDs) from mitochondrial and bacterial sources. The structure of a deletion mutant of Streptococcus sp. GlpO (GlpODelta, lacking a 50-residue insert that includes a flexible surface region) has been determined using multiwavelength anomalous dispersion data and refined at 2.3 A resolution. Using the GlpODelta structure as a search model, we have also determined the intact GlpO structure, as refined at 2.4 A resolution. The first two domains of the GlpO fold are most closely related to those of the flavoprotein glycine oxidase, where they function in FAD binding and substrate binding, respectively; the GlpO C-terminal domain consists of two helix bundles and is not closely related to any known structure. The flexible surface region in intact GlpO corresponds to a segment of missing electron density that links the substrate-binding domain to a betabetaalpha element of the FAD-binding domain. In accordance with earlier biochemical studies (stabilizations of the covalent FAD-N5-sulfite adduct and p-quinonoid form of 8-mercapto-FAD), Ile430-N, Thr431-N, and Thr431-OG are hydrogen bonded to FAD-O2alpha in GlpODelta, stabilizing the negative charge in these two modified flavins and facilitating transfer of a hydride to FAD-N5 (from Glp) as well. Active-site overlays with the glycine oxidase-N-acetylglycine and d-amino acid oxidase-d-alanine complexes demonstrate that Arg346 of GlpODelta is structurally equivalent to Arg302 and Arg285, respectively; in both cases, these residues interact directly with the amino acid substrate or inhibitor carboxylate. The structural and functional divergence between GlpO and the bacterial and mitochondrial GlpDs is also discussed.
Organizational Affiliation:
Center for Structural Biology, Wake Forest University School of Medicine, Winston-Salem, North Carolina 27157, USA.