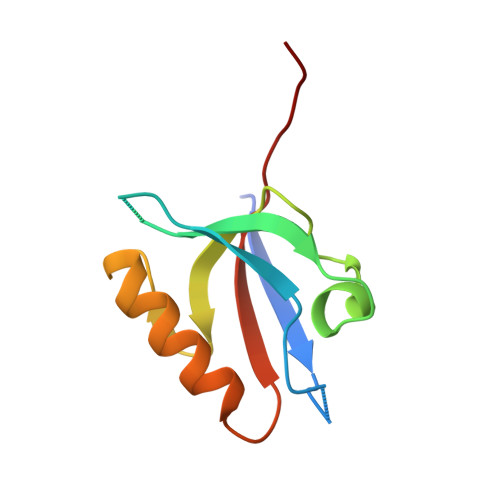
Crystal structure of the PDZ domains of human dishevelled 2 (homologous to Drosophila dsh).
Papagrigoriou, E., Gileadi, C., Elkins, J., Cooper, C., Ugochukwu, E., Turnbull, A., Pike, A.C.W., Gileadi, O., von Delft, F., Sundstrom, M., Arrowsmith, C.H., Weigelt, J., Edwards, A.M., Doyle, D.To be published.