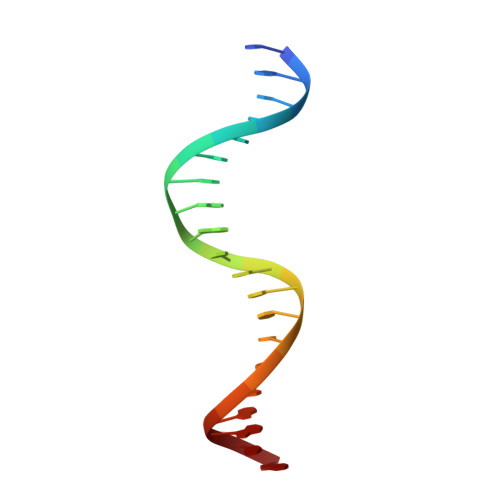
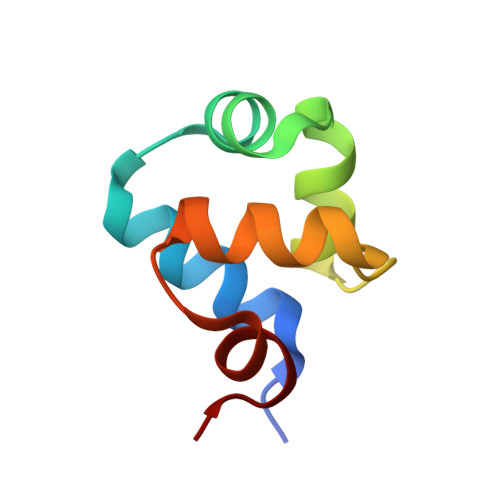P22 c2 repressor-operator complex: mechanisms of direct and indirect readout
Watkins, D., Hsiao, C., Woods, K.K., Koudelka, G.B., Williams, L.D.(2008) Biochemistry 47: 2325-2338
- PubMed: 18237194
- DOI: https://doi.org/10.1021/bi701826f
- Primary Citation of Related Structures:
2R1J - PubMed Abstract:
The P22 c2 repressor protein (P22R) binds to DNA sequence-specifically and helps to direct the temperate lambdoid bacteriophage P22 to the lysogenic developmental pathway. We describe the 1.6 A X-ray structure of the N-terminal domain (NTD) of P22R in a complex with a DNA fragment containing the synthetic operator sequence [d(ATTTAAGATATCTTAAAT)]2. This operator has an A-T base pair at position 9L and a T-A base pair at position 9R and is termed DNA9T. Direct readout: nondirectional van der Waals interactions between protein and DNA appear to confer sequence-specificity. The structure of the P22R NTD-DNA9T complex suggests that sequence-specificity arises substantially from lock-and-key interaction of a valine with a complementary binding cleft on the major groove surface of DNA9T. The cleft is formed by four methyl groups on sequential base pairs of 5'-TTAA-3'. The valine cleft is intrinsic to the DNA sequence and does not arise from protein-induced DNA conformational changes. Protein-DNA hydrogen bonding plays a secondary role in specificity. Indirect readout: it is known that the noncontacted bases in the center of the complex are important determinants of affinity. The protein induces a transition of the noncontacted region from B-DNA to B'-DNA. The B' state is characterized by a narrow minor groove and a zigzag spine of hydration. The free energy of the transition from B- to B'-DNA is known to depend on the sequence. Thus, the observed DNA conformation and hydration allows for the formulation of a predictive model of the indirect readout phenomenon.
Organizational Affiliation:
School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, Georgia 30332-0400, USA.