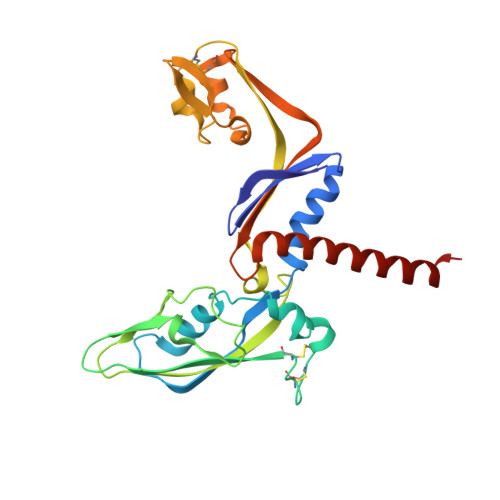
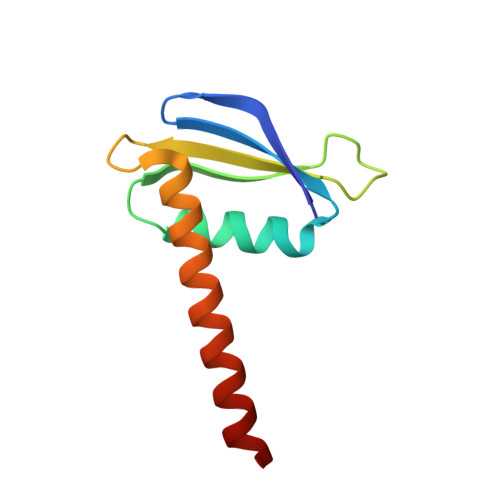
The X-ray crystal structure of RNA polymerase from Archaea.
Hirata, A., Klein, B.J., Murakami, K.S.(2008) Nature 451: 851-854
- PubMed: 18235446
- DOI: https://doi.org/10.1038/nature06530
- Primary Citation of Related Structures:
2PA8, 2PMZ, 3HKZ - PubMed Abstract:
The transcription apparatus in Archaea can be described as a simplified version of its eukaryotic RNA polymerase (RNAP) II counterpart, comprising an RNAPII-like enzyme as well as two general transcription factors, the TATA-binding protein (TBP) and the eukaryotic TFIIB orthologue TFB. It has been widely understood that precise comparisons of cellular RNAP crystal structures could reveal structural elements common to all enzymes and that these insights would be useful in analysing components of each enzyme that enable it to perform domain-specific gene expression. However, the structure of archaeal RNAP has been limited to individual subunits. Here we report the first crystal structure of the archaeal RNAP from Sulfolobus solfataricus at 3.4 A resolution, completing the suite of multi-subunit RNAP structures from all three domains of life. We also report the high-resolution (at 1.76 A) crystal structure of the D/L subcomplex of archaeal RNAP and provide the first experimental evidence of any RNAP possessing an iron-sulphur (Fe-S) cluster, which may play a structural role in a key subunit of RNAP assembly. The striking structural similarity between archaeal RNAP and eukaryotic RNAPII highlights the simpler archaeal RNAP as an ideal model system for dissecting the molecular basis of eukaryotic transcription.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, The Pennsylvania State University, University Park, Pennsylvania 16802, USA.