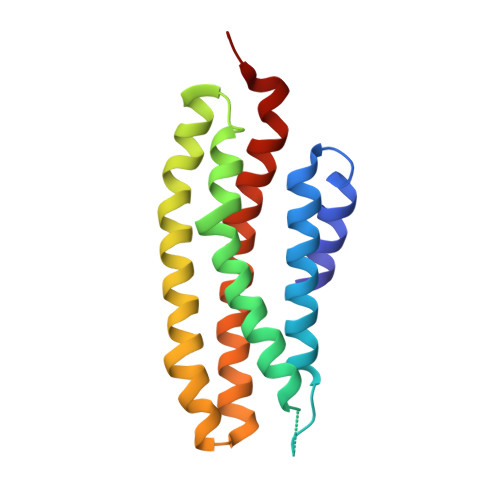
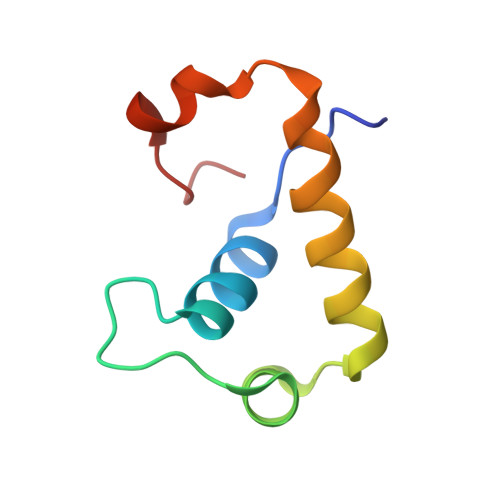
Crystal structure of the Escherichia coli regulator of sigma70, Rsd, in complex with sigma70 domain 4.
Patikoglou, G.A., Westblade, L.F., Campbell, E.A., Lamour, V., Lane, W.J., Darst, S.A.(2007) J Mol Biol 372: 649-659
- PubMed: 17681541
- DOI: https://doi.org/10.1016/j.jmb.2007.06.081
- Primary Citation of Related Structures:
2P7V - PubMed Abstract:
The Escherichia coli Rsd protein binds tightly and specifically to the RNA polymerase (RNAP) sigma(70) factor. Rsd plays a role in alternative sigma factor-dependent transcription by biasing the competition between sigma(70) and alternative sigma factors for the available core RNAP. Here, we determined the 2.6 A-resolution X-ray crystal structure of Rsd bound to sigma(70) domain 4 (sigma(70)(4)), the primary determinant for Rsd binding within sigma(70). The structure reveals that Rsd binding interferes with the two primary functions of sigma(70)(4), core RNAP binding and promoter -35 element binding. Interestingly, the most highly conserved Rsd residues form a network of interactions through the middle of the Rsd structure that connect the sigma(70)(4)-binding surface with three cavities exposed on distant surfaces of Rsd, suggesting functional coupling between sigma(70)(4) binding and other binding surfaces of Rsd, either for other proteins or for as yet unknown small molecule effectors. These results provide a structural basis for understanding the role of Rsd, as well as its ortholog, AlgQ, a positive regulator of Pseudomonas aeruginosa virulence, in transcription regulation.
Organizational Affiliation:
The Rockefeller University, Box 224, 1230 York Avenue, New York, NY 10065, USA.