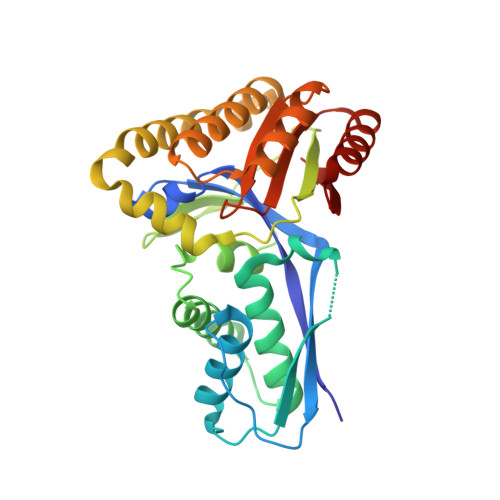
Crystal structure of the Streptococcus pneumoniae mevalonate kinase in complex with diphosphomevalonate.
Andreassi, J.L., Bilder, P.W., Vetting, M.W., Roderick, S.L., Leyh, T.S.(2007) Protein Sci 16: 983-989
- PubMed: 17400916
- DOI: https://doi.org/10.1110/ps.072755707
- Primary Citation of Related Structures:
2OI2 - PubMed Abstract:
Streptococcus pneumoniae, a ubiquitous gram-positive pathogen with an alarming, steadily evolving resistance to frontline antimicrobials, poses a severe global health threat both in the community and in the clinic. The recent discovery that diphosphomevalonate (DPM), an essential intermediate in the isoprenoid biosynthetic pathway, potently and allosterically inhibits S. pneumoniae mevalonate kinase (SpMK) without affecting the human isozyme established a new target and lead compound for antimicrobial design. Here we present the crystal structure of the first S. pneumoniae mevalonate kinase, at a resolution of 2.5 A and in complex with DPM.Mg(2+) in the active-site cleft. Structural comparison of SpMK with other members of the GHMP kinase family reveals that DPM functions as a partial bisubstrate analog (mevalonate linked to the pyrophosphoryl moiety of ATP) in that it elicits a ternary-complexlike form of the enzyme, except for localized disordering in a region that would otherwise interact with the missing portion of the nucleotide. Features of the SpMK-binding pockets are discussed in the context of established mechanistic findings and inherited human diseases linked to MK deficiency.
Organizational Affiliation:
DuPont Crop Protection, Stine-Haskell Research Center, Newark, Delaware 19711, USA.