Structural and kinetic properties of lumazine synthase isoenzymes in the order rhizobiales
Klinke, S., Zylberman, V., Bonomi, H.R., Haase, I., Guimaraes, B.G., Braden, B.C., Bacher, A., Fischer, M., Goldbaum, F.A.(2007) J Mol Biol 373: 664-680
- PubMed: 17854827
- DOI: https://doi.org/10.1016/j.jmb.2007.08.021
- Primary Citation of Related Structures:
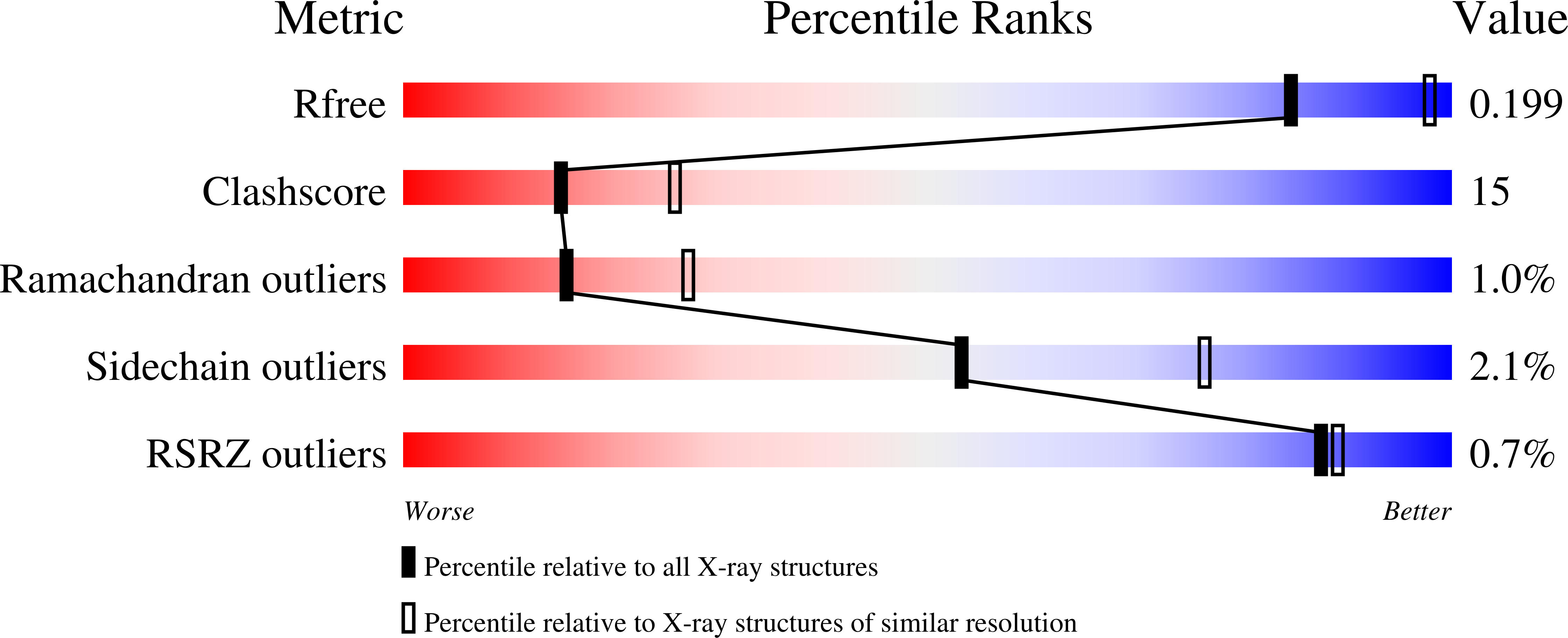
2F59, 2I0F, 2O6H, 2OBX - PubMed Abstract:
6,7-Dimethyl-8-ribityllumazine synthase (lumazine synthase; LS) catalyzes the penultimate step in the biosynthesis of riboflavin in plants and microorganisms. This protein is known to exhibit different quaternary assemblies between species, existing as free pentamers, decamers (dimers of pentamers) and icosahedrally arranged dodecamers of pentamers. A phylogenetic analysis on eubacterial, fungal and plant LSs allowed us to classify them into two categories: Type I LSs (pentameric or icosahedral) and Type II LSs (decameric). The Rhizobiales represent an order of alpha-proteobacteria that includes, among others, the genera Mesorhizobium, Agrobacterium and Brucella. Here, we present structural and kinetic studies on several LSs from Rhizobiales. Interestingly, Mesorhizobium and Brucella encode both a Type-I LS and a Type-II LS called RibH1 and RibH2, respectively. We show that Type II LSs appear to be almost inactive, whereas Type I LSs present a highly variable catalytic activity according to the genus. Additionally, we have solved four RibH1/RibH2 crystallographic structures from the genera Mesorhizobium and Brucella. The relationship between the active-site architecture and catalytic properties in these isoenzymes is discussed, and a model that describes the enzymatic behavior is proposed. Furthermore, sequence alignment studies allowed us to extend our results to the genus Agrobacterium. Our results suggest that the selective pressure controlling the riboflavin pathway favored the evolution of catalysts with low reaction rates, since the excess of flavins in the intracellular pool in Rhizobiales could act as a negative factor when these bacteria are exposed to oxidative or nitrosative stress.
Organizational Affiliation:
Fundación Instituto Leloir, IIBBA-CONICET, C1405BWE, Buenos Aires, Argentina. sklinke@leloir.org.ar