2NZU
Structural mechanism for the fine-tuning of CcpA function by the small molecule effectors G6P and FBP
- PDB DOI: https://doi.org/10.2210/pdb2NZU/pdb
- Classification: TRANSCRIPTION
- Organism(s): Priestia megaterium
- Expression System: Escherichia coli BL21(DE3)
- Mutation(s): Yes
- Deposited: 2006-11-25 Released: 2007-05-01
Experimental Data Snapshot
- Method: X-RAY DIFFRACTION
- Resolution: 2.50 Å
- R-Value Free: 0.274
- R-Value Work: 0.228
- R-Value Observed: 0.228
This is version 1.4 of the entry. See complete history.
Macromolecules
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Catabolite control protein | A [auth G] | 280 | Priestia megaterium | Mutation(s): 0 Gene Names: ccpA | 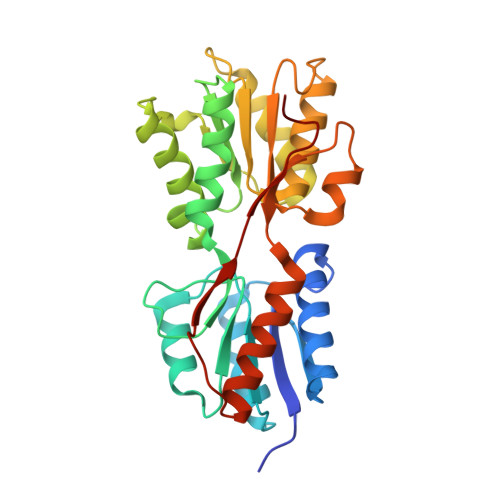 |
UniProt | |||||
Find proteins for P46828 (Priestia megaterium) Explore P46828 Go to UniProtKB: P46828 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P46828 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by:
(by identity cutoff) | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Phosphocarrier protein HPr | B [auth L] | 88 | Priestia megaterium | Mutation(s): 1 Gene Names: ptsH | 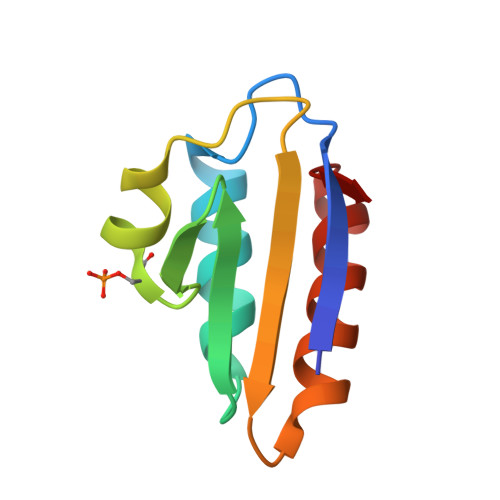 |
UniProt | |||||
Find proteins for O69250 (Priestia megaterium) Explore O69250 Go to UniProtKB: O69250 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O69250 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Small Molecules
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| BG6 Query on BG6 | C [auth G] | 6-O-phosphono-beta-D-glucopyranose C6 H13 O9 P NBSCHQHZLSJFNQ-VFUOTHLCSA-N |  | ||
| SO4 Query on SO4 | D [auth G] E [auth G] F [auth G] G H [auth G] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| SEP Query on SEP | B [auth L] | L-PEPTIDE LINKING | C3 H8 N O6 P |  | SER |
Experimental Data & Validation
Experimental Data
- Method: X-RAY DIFFRACTION
- Resolution: 2.50 Å
- R-Value Free: 0.274
- R-Value Work: 0.228
- R-Value Observed: 0.228
- Space Group: P 41 21 2
Unit Cell:
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 68.63 | α = 90 |
| b = 68.63 | β = 90 |
| c = 230.251 | γ = 90 |
| Software Name | Purpose |
|---|---|
| CNS | refinement |
| ADSC | data collection |
| MOSFLM | data reduction |
| CCP4 | data scaling |
| MOLREP | phasing |
Entry History
Deposition Data
- Released Date: 2007-05-01 Deposition Author(s): Schumacher, M.A., Hillen, W., Brennan, R.G.
Revision History (Full details and data files)
- Version 1.0: 2007-05-01
Type: Initial release - Version 1.1: 2008-05-01
Changes: Version format compliance - Version 1.2: 2011-07-13
Changes: Derived calculations, Version format compliance - Version 1.3: 2020-07-29
Type: Remediation
Reason: Carbohydrate remediation
Changes: Data collection, Database references, Derived calculations, Structure summary - Version 1.4: 2023-08-30
Changes: Data collection, Database references, Refinement description, Structure summary













