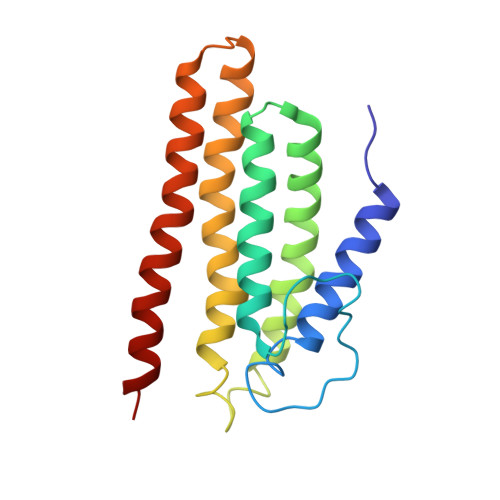
Structure and Function of the PriC DNA Replication Restart Protein.
Wessel, S.R., Cornilescu, C.C., Cornilescu, G., Metz, A., Leroux, M., Hu, K., Sandler, S.J., Markley, J.L., Keck, J.L.(2016) J Biol Chem 291: 18384-18396
- PubMed: 27382050
- DOI: https://doi.org/10.1074/jbc.M116.738781
- Primary Citation of Related Structures:
2NCJ - PubMed Abstract:
Collisions between DNA replication complexes (replisomes) and barriers such as damaged DNA or tightly bound protein complexes can dissociate replisomes from chromosomes prematurely. Replisomes must be reloaded under these circumstances to avoid incomplete replication and cell death. Bacteria have evolved multiple pathways that initiate DNA replication restart by recognizing and remodeling abandoned replication forks and reloading the replicative helicase. In vitro, the simplest of these pathways is mediated by the single-domain PriC protein, which, along with the DnaC helicase loader, can load the DnaB replicative helicase onto DNA bound by the single-stranded DNA (ssDNA)-binding protein (SSB). Previous biochemical studies have identified PriC residues that mediate interactions with ssDNA and SSB. However, the mechanisms by which PriC drives DNA replication restart have remained poorly defined due to the limited structural information available for PriC. Here, we report the NMR structure of full-length PriC from Cronobacter sakazakii PriC forms a compact bundle of α-helices that brings together residues involved in ssDNA and SSB binding at adjacent sites on the protein surface. Disruption of these interaction sites and of other conserved residues leads to decreased DnaB helicase loading onto SSB-bound DNA. We also demonstrate that PriC can directly interact with DnaB and the DnaB·DnaC complex. These data lead to a model in which PriC acts as a scaffold for recruiting DnaB·DnaC to SSB/ssDNA sites present at stalled replication forks.
Organizational Affiliation:
From the Department of Biomolecular Chemistry, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin 53706.