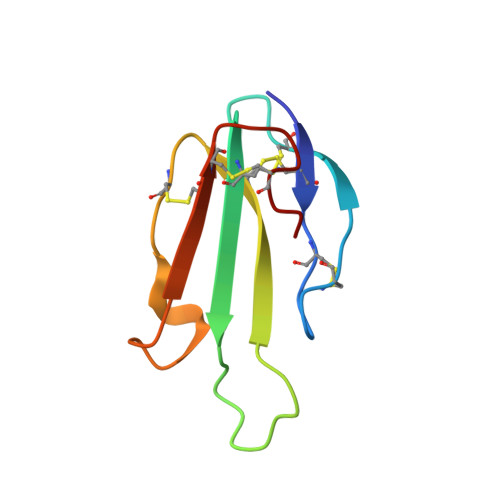
Secreted Isoform of Human Lynx1 (SLURP-2): Spatial Structure and Pharmacology of Interactions with Different Types of Acetylcholine Receptors.
Lyukmanova, E.N., Shulepko, M.A., Shenkarev, Z.O., Bychkov, M.L., Paramonov, A.S., Chugunov, A.O., Kulbatskii, D.S., Arvaniti, M., Dolejsi, E., Schaer, T., Arseniev, A.S., Efremov, R.G., Thomsen, M.S., Dolezal, V., Bertrand, D., Dolgikh, D.A., Kirpichnikov, M.P.(2016) Sci Rep 6: 30698-30698
- PubMed: 27485575
- DOI: https://doi.org/10.1038/srep30698
- Primary Citation of Related Structures:
2N99 - PubMed Abstract:
Human-secreted Ly-6/uPAR-related protein-2 (SLURP-2) regulates the growth and differentiation of epithelial cells. Previously, the auto/paracrine activity of SLURP-2 was considered to be mediated via its interaction with the α3β2 subtype of the nicotinic acetylcholine receptors (nAChRs). Here, we describe the structure and pharmacology of a recombinant analogue of SLURP-2. Nuclear magnetic resonance spectroscopy revealed a 'three-finger' fold of SLURP-2 with a conserved β-structural core and three protruding loops. Affinity purification using cortical extracts revealed that SLURP-2 could interact with the α3, α4, α5, α7, β2, and β4 nAChR subunits, revealing its broader pharmacological profile. SLURP-2 inhibits acetylcholine-evoked currents at α4β2 and α3β2-nAChRs (IC50 ~0.17 and >3 μM, respectively) expressed in Xenopus oocytes. In contrast, at α7-nAChRs, SLURP-2 significantly enhances acetylcholine-evoked currents at concentrations <1 μM but induces inhibition at higher concentrations. SLURP-2 allosterically interacts with human M1 and M3 muscarinic acetylcholine receptors (mAChRs) that are overexpressed in CHO cells. SLURP-2 was found to promote the proliferation of human oral keratinocytes via interactions with α3β2-nAChRs, while it inhibited cell growth via α7-nAChRs. SLURP-2/mAChRs interactions are also probably involved in the control of keratinocyte growth. Computer modeling revealed possible SLURP-2 binding to the 'classical' orthosteric agonist/antagonist binding sites at α7 and α3β2-nAChRs.
Organizational Affiliation:
Lomonosov Moscow State University, Leninskie Gori 1, Moscow 119234, Russian Federation.