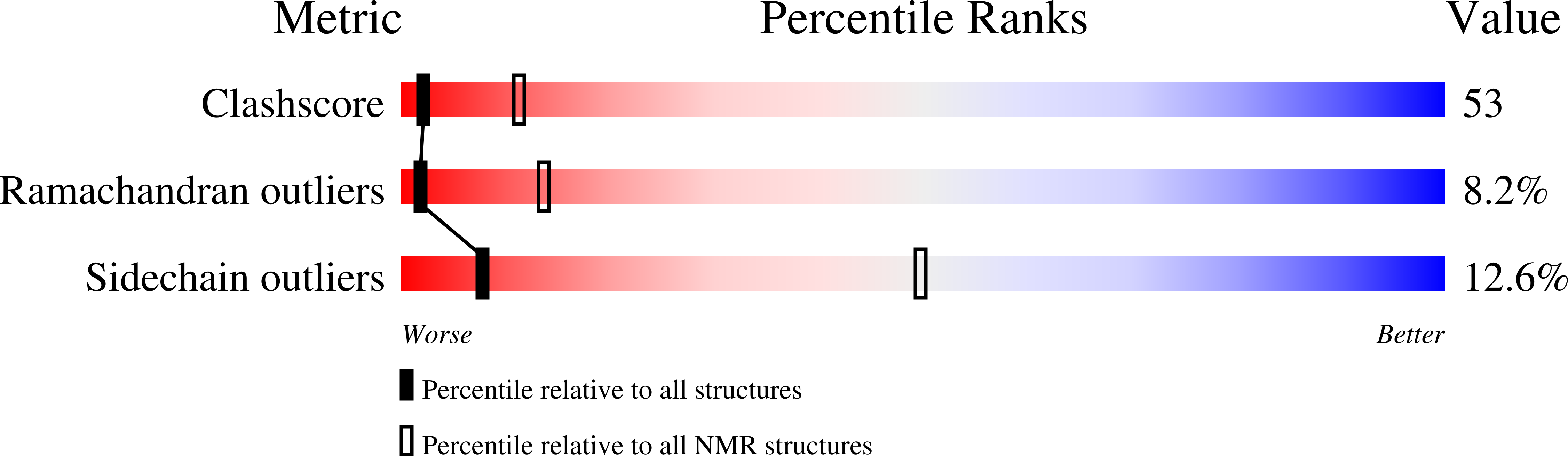A novel lipid transfer protein from the dill Anethum graveolens L.: isolation, structure, heterologous expression, and functional characteristics.
Melnikova, D.N., Mineev, K.S., Finkina, E.I., Arseniev, A.S., Ovchinnikova, T.V.(2016) J Pept Sci 22: 59-66
- PubMed: 26680443
- DOI: https://doi.org/10.1002/psc.2840
- Primary Citation of Related Structures:
2N2Z - PubMed Abstract:
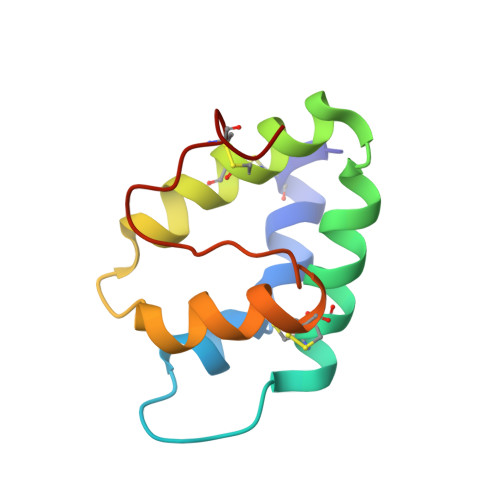
A novel lipid transfer protein, designated as Ag-LTP, was isolated from aerial parts of the dill Anethum graveolens L. Structural, antimicrobial, and lipid binding properties of the protein were studied. Complete amino acid sequence of Ag-LTP was determined. The protein has molecular mass of 9524.4 Da, consists of 93 amino acid residues including eight cysteines forming four disulfide bonds. The recombinant Ag-LTP was overexpressed in Escherichia coli and purified. NMR investigation shows that the Ag-LTP spatial structure contains four α-helices, forming the internal hydrophobic cavity, and a long C-terminal tail. The measured volume of the Ag-LTP hydrophobic cavity is equal to ~800 A(3), which is much larger than those of other plant LTP1s. Ag-LTP has weak antifungal activity and unpronounced lipid binding specificity but effectively binds plant hormone jasmonic acid. Our results afford further molecular insight into biological functions of LTP in plants.
Organizational Affiliation:
Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Miklukho-Maklaya str., 16/10, 117997, Moscow, Russia.