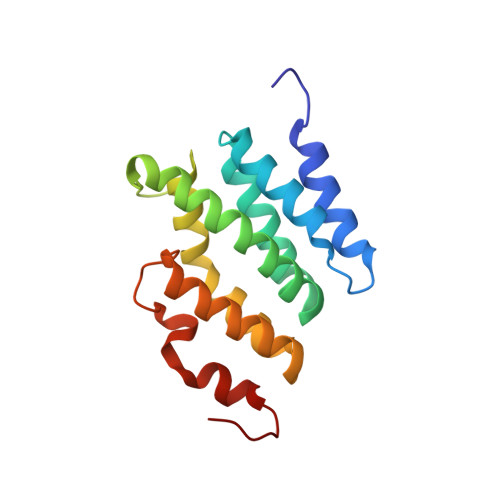
Molecular Basis for Coordinating Transcription Termination with Noncoding RNA Degradation.
Tudek, A., Porrua, O., Kabzinski, T., Lidschreiber, M., Kubicek, K., Fortova, A., Lacroute, F., Vanacova, S., Cramer, P., Stefl, R., Libri, D.(2014) Mol Cell 55: 467-481
- PubMed: 25066235
- DOI: https://doi.org/10.1016/j.molcel.2014.05.031
- Primary Citation of Related Structures:
2MOW - PubMed Abstract:
The Nrd1-Nab3-Sen1 (NNS) complex is essential for controlling pervasive transcription and generating sn/snoRNAs in S. cerevisiae. The NNS complex terminates transcription of noncoding RNA genes and promotes exosome-dependent processing/degradation of the released transcripts. The Trf4-Air2-Mtr4 (TRAMP) complex polyadenylates NNS target RNAs and favors their degradation. NNS-dependent termination and degradation are coupled, but the mechanism underlying this coupling remains enigmatic. Here we provide structural and functional evidence demonstrating that the same domain of Nrd1p interacts with RNA polymerase II and Trf4p in a mutually exclusive manner, thus defining two alternative forms of the NNS complex, one involved in termination and the other in degradation. We show that the Nrd1-Trf4 interaction is required for optimal exosome activity in vivo and for the stimulation of polyadenylation of NNS targets by TRAMP in vitro. We propose that transcription termination and RNA degradation are coordinated by switching between two alternative partners of the NNS complex.
Organizational Affiliation:
Centre de Génétique Moléculaire, CNRS UPR3404, 91190 Gif sur Yvette, France.