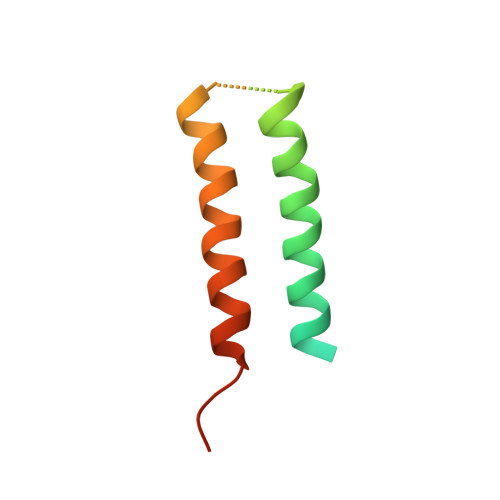
Structure of CrgA, a cell division structural and regulatory protein from Mycobacterium tuberculosis, in lipid bilayers.
Das, N., Dai, J., Hung, I., Rajagopalan, M.R., Zhou, H.X., Cross, T.A.(2015) Proc Natl Acad Sci U S A 112: E119-E126
- PubMed: 25548160
- DOI: https://doi.org/10.1073/pnas.1415908112
- Primary Citation of Related Structures:
2MMU - PubMed Abstract:
The 93-residue transmembrane protein CrgA in Mycobacterium tuberculosis is a central component of the divisome, a large macromolecular machine responsible for cell division. Through interactions with multiple other components including FtsZ, FtsQ, FtsI (PBPB), PBPA, and CwsA, CrgA facilitates the recruitment of the proteins essential for peptidoglycan synthesis to the divisome and stabilizes the divisome. CrgA is predicted to have two transmembrane helices. Here, the structure of CrgA was determined in a liquid-crystalline lipid bilayer environment by solid-state NMR spectroscopy. Oriented-sample data yielded orientational restraints, whereas magic-angle spinning data yielded interhelical distance restraints. These data define a complete structure for the transmembrane domain and provide rich information on the conformational ensembles of the partially disordered N-terminal region and interhelical loop. The structure of the transmembrane domain was refined using restrained molecular dynamics simulations in an all-atom representation of the same lipid bilayer environment as in the NMR samples. The two transmembrane helices form a left-handed packing arrangement with a crossing angle of 24° at the conserved Gly39 residue. This helix pair exposes other conserved glycine and alanine residues to the fatty acyl environment, which are potential sites for binding CrgA's partners such as CwsA and FtsQ. This approach combining oriented-sample and magic-angle spinning NMR spectroscopy in native-like lipid bilayers with restrained molecular dynamics simulations represents a powerful tool for structural characterization of not only isolated membrane proteins, but their complexes, such as those that form macromolecular machines.
Organizational Affiliation:
Institute of Molecular Biophysics, and National High Magnetic Field Laboratory, Florida State University, Tallahassee, FL 32310; and.