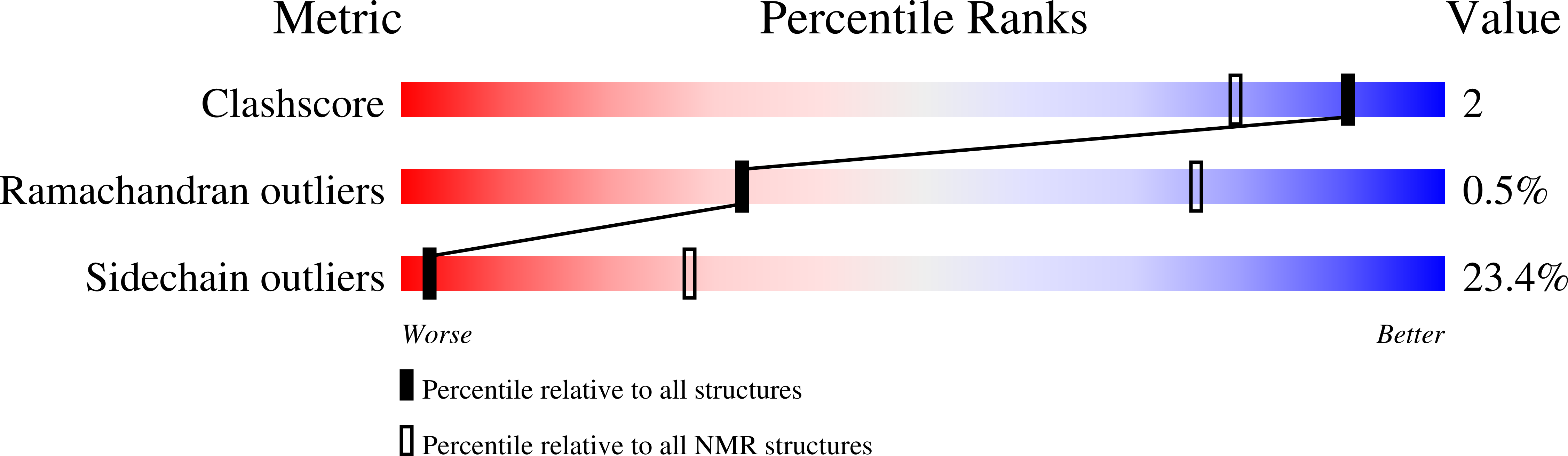Relationships between IgE/IgG4 epitopes, structure and function in Anisakis simplex Ani s 5, a member of the SXP/RAL-2 protein family
Garcia-Mayoral, M.F., Trevino, M.A., Perez-Pinar, T., Caballero, M.L., Knaute, T., Umpierrez, A., Bruix, M., Rodriguez-Perez, R.(2014) PLoS Negl Trop Dis 8: e2735-e2735
- PubMed: 24603892
- DOI: https://doi.org/10.1371/journal.pntd.0002735
- Primary Citation of Related Structures:
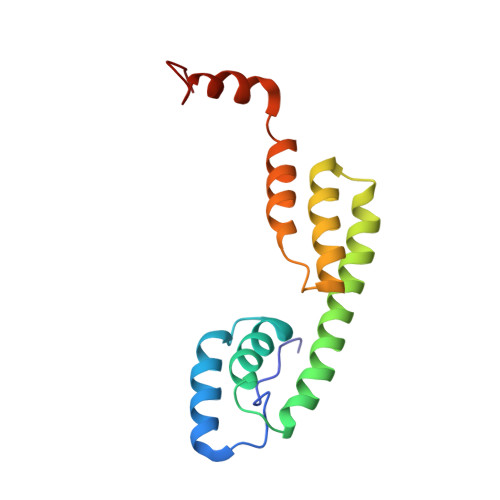
2MAR - PubMed Abstract:
Anisakiasis is a re-emerging global disease caused by consumption of raw or lightly cooked fish contaminated with L3 Anisakis larvae. This zoonotic disease is characterized by severe gastrointestinal and/or allergic symptoms which may misdiagnosed as appendicitis, gastric ulcer or other food allergies. The Anisakis allergen Ani s 5 is a protein belonging to the SXP/RAL-2 family; it is detected exclusively in nematodes. Previous studies showed that SXP/RAL-2 proteins are active antigens; however, their structure and function remain unknown. The aim of this study was to elucidate the three-dimensional structure of Ani s 5 and its main IgE and IgG4 binding regions.
Organizational Affiliation:
Institute of Physical Chemistry "Rocasolano". CSIC. Madrid, Spain.