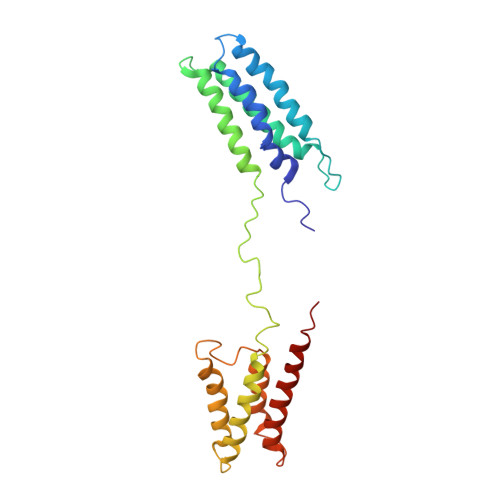
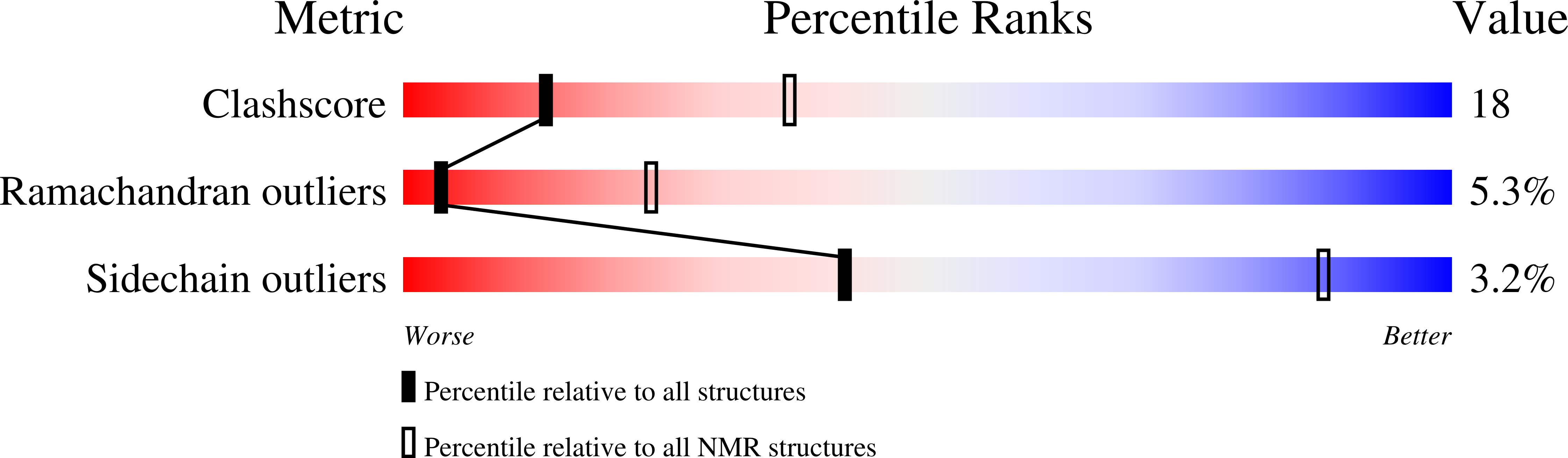Flexible IgE epitope-containing domains of Phl p 5 cause high allergenic activity.
Gobl, C., Focke-Tejkl, M., Najafi, N., Schrank, E., Madl, T., Kosol, S., Madritsch, C., Dorofeeva, Y., Flicker, S., Thalhamer, J., Valenta, R., Zangger, K., Tjandra, N.(2017) J Allergy Clin Immunol 140: 1187-1191
- PubMed: 28532654
- DOI: https://doi.org/10.1016/j.jaci.2017.05.005
- Primary Citation of Related Structures:
2M64 - PubMed Abstract:
This is the first study determining the three-dimensional structure of Phl p 5a which reveals a novel mechanism for high allergenic activity based on flexibly connected IgE-reactive domains which cross-link effector cell-bound IgE more efficiently than isolated rigid globular proteins. These findings may also form a basis for specific immunotherapy strategies.
Organizational Affiliation:
Institute of Chemistry/Organic and Bioorganic Chemistry, University of Graz, Graz, Austria; Institute of Structural Biology, Helmholtz Zentrum München, Neuherberg, Germany; Biomolecular NMR, Department of Chemistry, Technische Universität München, Garching, Germany; Laboratory of Molecular Biophysics, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Md.