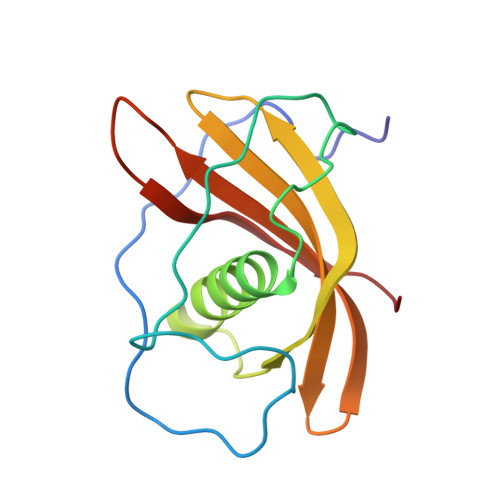
Resonance assignments and secondary structure of a phytocystatin from Ananas comosus
Irene, D., Chen, B.J., Lo, S.H., Liu, T.H., Tzen, J.T.C., Chyan, C.L.(2012) Biomol NMR Assign 6: 99-101
- PubMed: 21814766
- DOI: https://doi.org/10.1007/s12104-011-9334-1
- Primary Citation of Related Structures:
2L4V - PubMed Abstract:
A cDNA encoding a cysteine protease inhibitor, cystatin was cloned from pineapple (Ananas comosus L.) stem. This clone was constructed into an expression vector and expressed in E. coli and purified to homogeneous. The recombinant pineapple cystatins (AcCYS) showed effectively inhibitory activity toward cysteine proteases including papain, bromelain, and cathepsin B. In order to unravel its inhibitory action from structural point of view, multidimensional heteronuclear NMR techniques were used to characterize the structure of AcCYS. The full (1)H, (15)N, and (13)C resonance assignments of AcCYS were determined. The secondary structure of AcCYS was identified by using the assigned chemical shift of (1)Hα, (13)Cα, (13)Cβ, and (13)CO through the consensus chemical shift index (CSI). The results of CSI analysis suggest 5 β-strands (residues 45-47, 84-91, 94-104, 106-117, and 123-130) and one α-helix (residues 55-73).
Organizational Affiliation:
Department of Chemistry, National Dong Hwa University, Shoufeng, Hualien, 974 Taiwan, ROC.