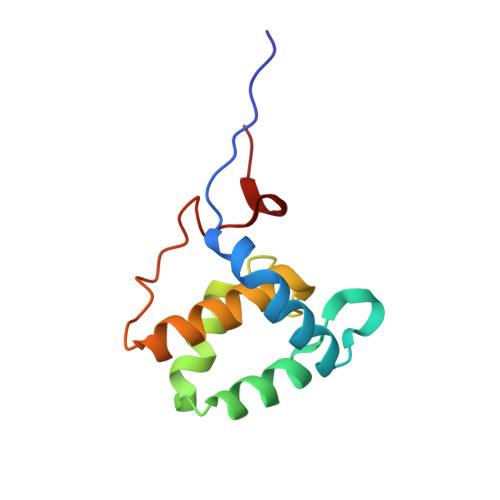
Mechanism for the selective interaction of C-terminal Eps15 homology domain proteins with specific Asn-Pro-Phe-containing partners.
Kieken, F., Sharma, M., Jovic, M., Giridharan, S.S., Naslavsky, N., Caplan, S., Sorgen, P.L.(2010) J Biol Chem 285: 8687-8694
- PubMed: 20106972
- DOI: https://doi.org/10.1074/jbc.M109.045666
- PubMed Abstract:
Epidermal growth factor receptor tyrosine kinase substrate 15 (Eps15) homology (EH)-domain proteins can be divided into two classes: those with an N-terminal EH-domain(s), and the C-terminal Eps15 homology domain-containing proteins (EHDs). Whereas many N-terminal EH-domain proteins regulate internalization events, the best characterized C-terminal EHD, EHD1, regulates endocytic recycling. Because EH-domains interact with the tripeptide Asn-Pro-Phe (NPF), it is of critical importance to elucidate the molecular mechanisms that allow EHD1 and its paralogs to interact selectively with a subset of the hundreds of NPF-containing proteins expressed in mammalian cells. Here, we capitalize on our findings that C-terminal EH-domains possess highly positively charged interaction surfaces and that many NPF-containing proteins that interact with C-terminal (but not N-terminal) EH-domains are followed by acidic residues. Using the recently identified EHD1 interaction partner molecule interacting with CasL (MICAL)-Like 1 (MICAL-L1) as a model, we have demonstrated that only the first of its two NPF motifs is required for EHD1 binding. Because only this first NPF is followed by acidic residues, we have utilized glutathione S-transferase pulldowns, two-hybrid analysis, and NMR to demonstrate that the flanking acidic residues "fine tune" the binding affinity to EHD1. Indeed, our NMR solution structure of the EHD1 EH-domain in complex with the MICAL-L1 NPFEEEEED peptide indicates that the first two flanking Glu residues lie in a position favorable to form salt bridges with Lys residues within the EH-domain. Our data provide a novel explanation for the selective interaction of C-terminal EH-domains with specific NPF-containing proteins and allow for the prediction of new interaction partners with C-terminal EHDs.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology and Eppley Cancer Center, University of Nebraska Medical Center, Omaha, Nebraska 68198-5870, USA.