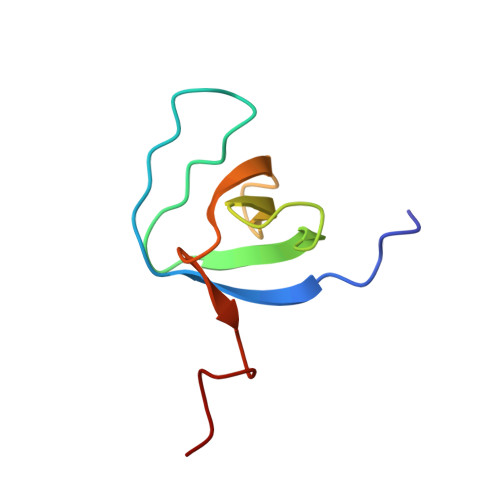
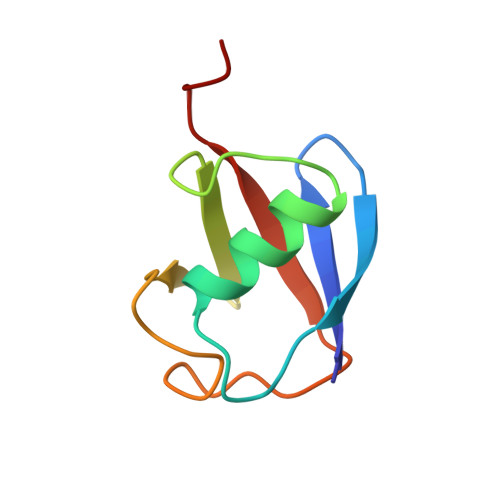
Structural Basis for Ubiquitin Recognition by SH3 Domains
He, Y., Hicke, L., Radhakrishnan, I.(2007) J Mol Biol 373: 190-196
- PubMed: 17765920
- DOI: https://doi.org/10.1016/j.jmb.2007.07.074
- Primary Citation of Related Structures:
2JT4 - PubMed Abstract:
The SH3 domain is a protein-protein interaction module commonly found in intracellular signaling and adaptor proteins. The SH3 domains of multiple endocytic proteins have been recently implicated in binding ubiquitin, which serves as a signal for diverse cellular processes including gene regulation, endosomal sorting, and protein destruction. Here we describe the solution NMR structure of ubiquitin in complex with an SH3 domain belonging to the yeast endocytic protein Sla1. The ubiquitin binding surface of the Sla1 SH3 domain overlaps substantially with the canonical binding surface for proline-rich ligands. Like many other ubiquitin-binding motifs, the SH3 domain engages the Ile44 hydrophobic patch of ubiquitin. A phenylalanine residue located at the heart of the ubiquitin-binding surface of the SH3 domain serves as a key specificity determinant. The structure of the SH3-ubiquitin complex explains how a subset of SH3 domains has acquired this non-traditional function.
Organizational Affiliation:
Department of Biochemistry, Molecular Biology and Cell Biology, Northwestern University, Evanston, IL 60208-3500, USA.