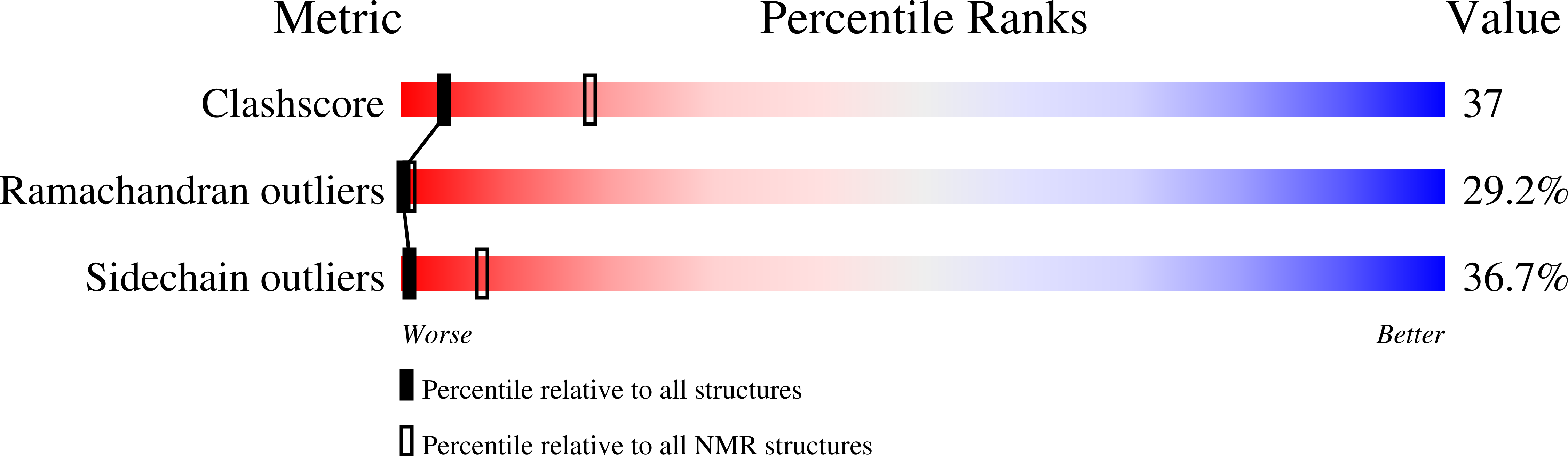Obestatin conformational features: a strategy to unveil obestatin's biological role?
Scrima, M., Campiglia, P., Esposito, C., Gomez-Monterrey, I., Novellino, E., D'Ursi, A.M.(2007) Biochem Biophys Res Commun 363: 500-505
- PubMed: 17904104
- DOI: https://doi.org/10.1016/j.bbrc.2007.08.200
- Primary Citation of Related Structures:
2JSH, 2JSI, 2JSJ - PubMed Abstract:
Obestatin and its derivative Ob(11-23) are recently discovered peptides produced in the rat stomach. They have proven to be involved in the regulation of energy balance, inhibiting feeding, causing reductions in food intake, body weight and jejunal contraction in rodents. The G-protein coupled receptor, GPR39, was originally proposed as being an obestatin target receptor, but this remains controversial. As such, the molecular mechanism for obestatin's effects in vivo is still uncertain. Here we report the CD and NMR conformational analysis of obestatin and Ob(11-23). Both peptides assume a regular secondary structure in the C-terminal region of the molecule. In this region, structural elements similar to other GPCR binding neuropeptides support the identity of obestatin as a new and functionally autonomous GPCR ligand. Conversely sequence and conformational specificity point to a new farmacoforic structure, on which innovative derivatives with a potential role in the treatment of obesity can be designed and synthetized.
Organizational Affiliation:
Department of Pharmaceutical Sciences, University of Salerno, I-84084 Fisciano, Italy.