Structural and Enzymatic Investigation of the Sulfolobus Solfataricus Uridylate Kinase Shows Competitive Utp Inhibition and the Lack of GTP Stimulation
Jensen, K.S., Johansson, E., Jensen, K.F.(2007) Biochemistry 46: 2745
- PubMed: 17297917
- DOI: https://doi.org/10.1021/bi0618159
- Primary Citation of Related Structures:
2J4J, 2J4K, 2J4L - PubMed Abstract:
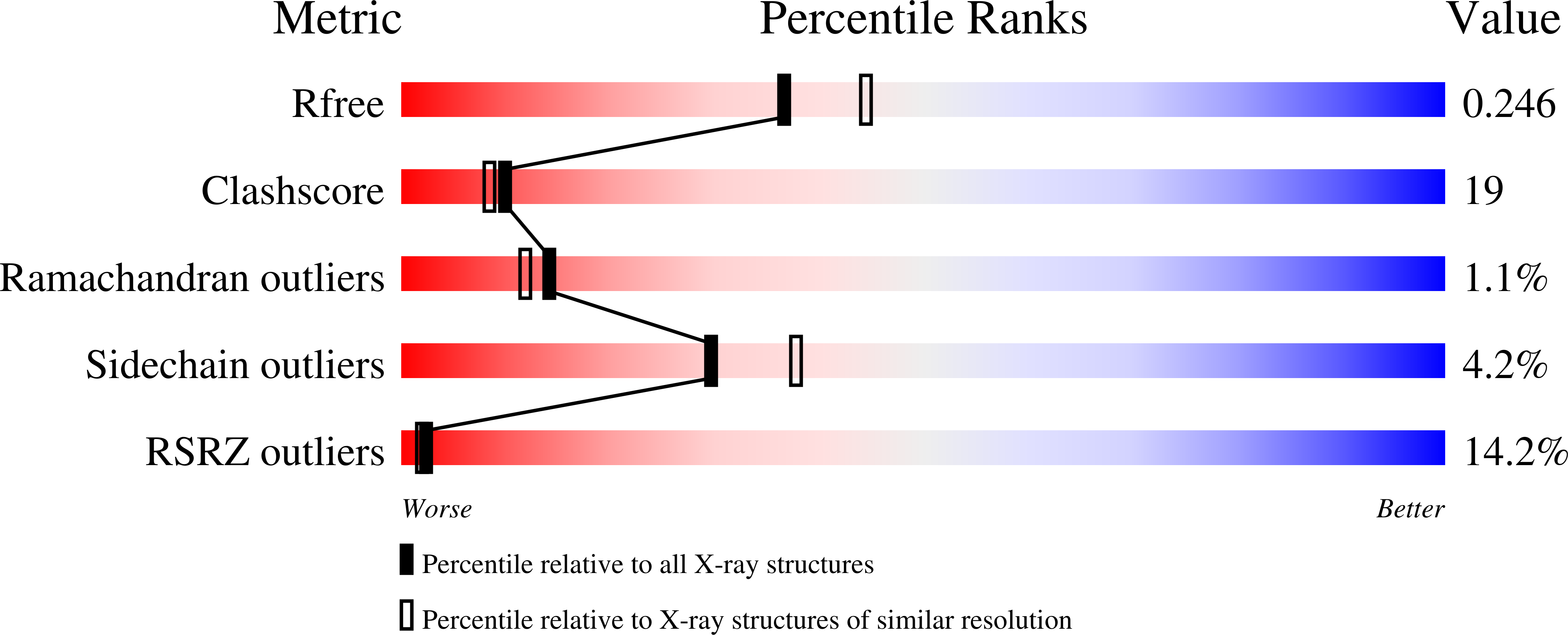
The pyrH gene encoding uridylate kinase (UMPK) from the extreme thermoacidophilic archaeon Sulfolobus solfataricus was cloned and expressed in Escherichia coli, and the enzyme (SsUMPK) was purified. Size exclusion chromatography and sedimentation experiments showed that the oligomeric state in solution is hexameric. SsUMPK shows maximum catalytic rate at pH 7.0, and variation of pH only influences the turnover number. Catalysis proceeds by a sequential reaction mechanism of random order and depends on a divalent cation. The enzyme exhibits high substrate specificity toward UMP and ATP and is inhibited by UTP, whereas CTP and GTP do not influence activity. UTP binds to the enzyme with a sigmoid binding curve, whereas GTP does not bind. The crystal structure of SsUMPK was determined for three different complexes, a ternary complex with UMP and the nonhydrolyzable ATP analogue beta,gamma-methylene-ATP, a complex with UMP, and a complex with UTP to 2.1, 2.2, and 2.8 A resolution, respectively. One UTP molecule was bound in the acceptor site per subunit, leading to the exclusion of both substrates from the active site. In all cases, SsUMPK crystallized as a hexamer with the main fold shared with other prokaryotic UMPKs. Similar to UMPK from Pyrococcus furiosus, SsUMPK has an active site enclosing loop. This loop was only ordered in one subunit in the ternary complex, which also contained an unusual arrangement of ligands (possibly a dinucleotide) in the active site and an altered orientation of the catalytic residue Arg48 relative to the other five subunits of the hexamer.
Organizational Affiliation:
Department of Biological Chemistry, Institute of Molecular Biology, University of Copenhagen, Sølvgade 83H, DK-1307 Copenhagen K, Denmark.