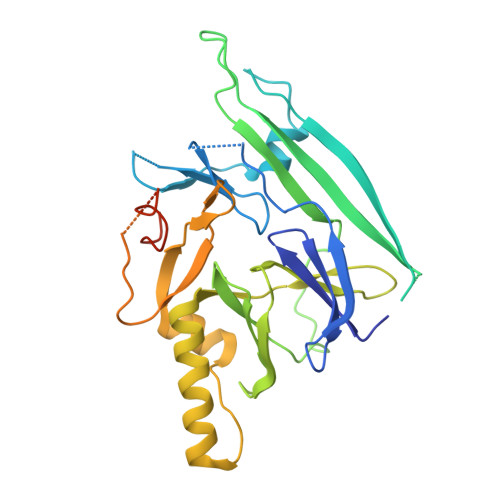
The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response.
Zhou, J., Liu, C.Y., Back, S.H., Clark, R.L., Peisach, D., Xu, Z., Kaufman, R.J.(2006) Proc Natl Acad Sci U S A 103: 14343-14348
- PubMed: 16973740
- DOI: https://doi.org/10.1073/pnas.0606480103
- Primary Citation of Related Structures:
2HZ6 - PubMed Abstract:
The unfolded protein response (UPR) is an evolutionarily conserved mechanism by which all eukaryotic cells adapt to the accumulation of unfolded proteins in the endoplasmic reticulum (ER). Inositol-requiring kinase 1 (IRE1) and PKR-related ER kinase (PERK) are two type I transmembrane ER-localized protein kinase receptors that signal the UPR through a process that involves homodimerization and autophosphorylation. To elucidate the molecular basis of the ER transmembrane signaling event, we determined the x-ray crystal structure of the luminal domain of human IRE1alpha. The monomer of the luminal domain comprises a unique fold of a triangular assembly of beta-sheet clusters. Structural analysis identified an extensive dimerization interface stabilized by hydrogen bonds and hydrophobic interactions. Dimerization creates an MHC-like groove at the interface. However, because this groove is too narrow for peptide binding and the purified luminal domain forms high-affinity dimers in vitro, peptide binding to this groove is not required for dimerization. Consistent with our structural observations, mutations that disrupt the dimerization interface produced IRE1alpha molecules that failed to either dimerize or activate the UPR upon ER stress. In addition, mutations in a structurally homologous region within PERK also prevented dimerization. Our structural, biochemical, and functional studies in vivo altogether demonstrate that IRE1 and PERK have conserved a common molecular interface necessary and sufficient for dimerization and UPR signaling.
Organizational Affiliation:
Life Sciences Institute, LSI 3163B, Department of Biological Chemistry, University of Michigan Medical Center, 210 Washtenaw Avenue, Ann Arbor, MI 48109, USA.