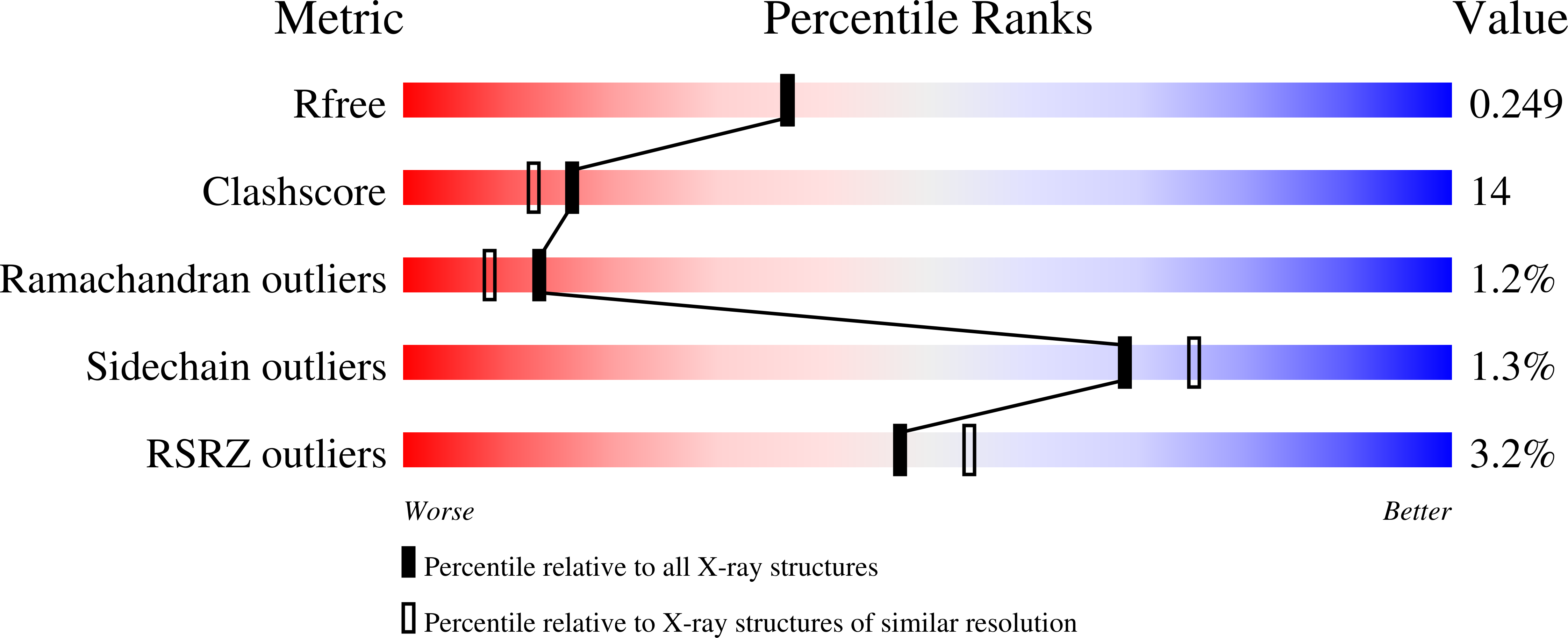
Structural characterization of CA1462, the Candida albicans thiamine pyrophosphokinase.
Santini, S., Monchois, V., Mouz, N., Sigoillot, C., Rousselle, T., Claverie, J.M., Abergel, C.(2008) BMC Struct Biol 8: 33-33
- PubMed: 18652651
- DOI: https://doi.org/10.1186/1472-6807-8-33
- Primary Citation of Related Structures:
2G9Z, 2HH9 - PubMed Abstract:
In search of new antifungal targets of potential interest for pharmaceutical companies, we initiated a comparative genomics study to identify the most promising protein-coding genes in fungal genomes. One criterion was the protein sequence conservation between reference pathogenic genomes. A second criterion was that the corresponding gene in Saccharomyces cerevisiae should be essential. Since thiamine pyrophosphate is an essential product involved in a variety of metabolic pathways, proteins responsible for its production satisfied these two criteria.
Organizational Affiliation:
Information Genomique et Structurale, UPR2589, Parc Scientifique de Luminy, 13288, Marseille cedex 09, France. santini.s@fsagx.ac.be