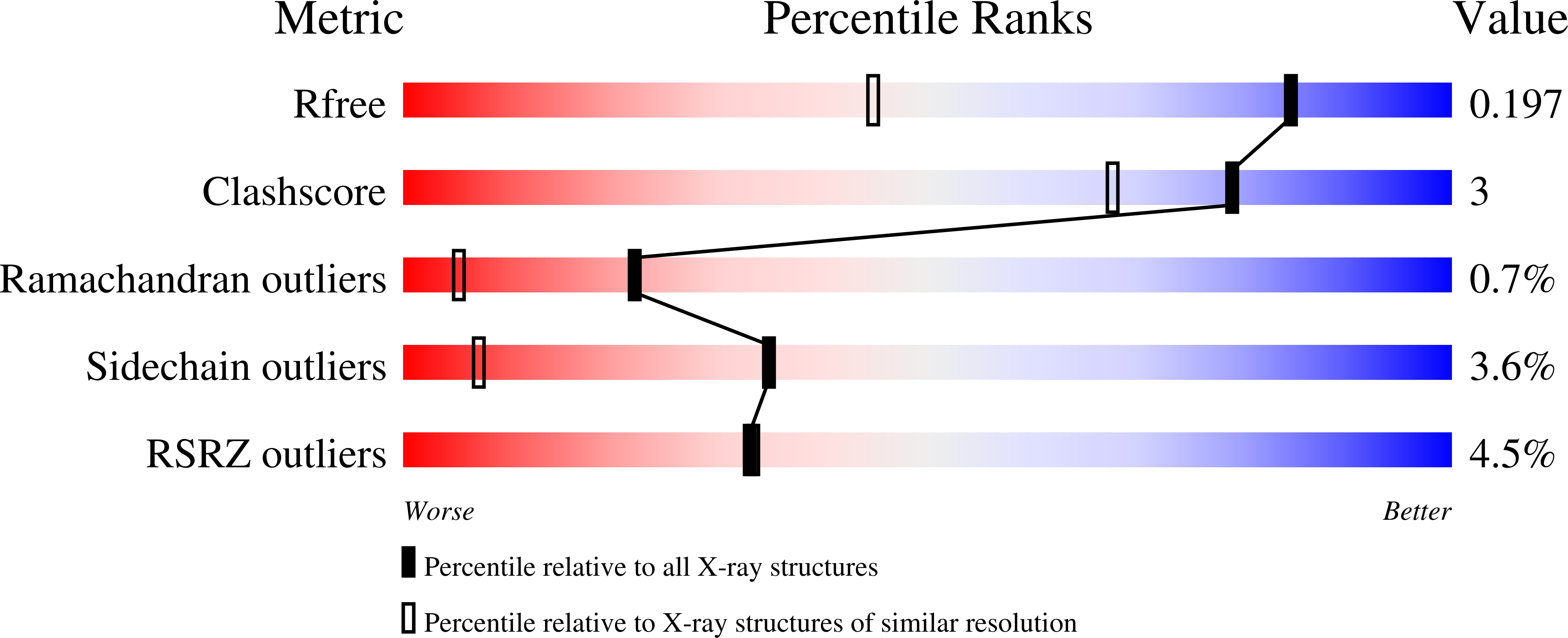
The crystal structures of the psychrophilic subtilisin S41 and the mesophilic subtilisin Sph reveal the same calcium-loaded state.
Almog, O., Gonzalez, A., Godin, N., de Leeuw, M., Mekel, M.J., Klein, D., Braun, S., Shoham, G., Walter, R.L.(2009) Proteins 74: 489-496
- PubMed: 18655058
- DOI: https://doi.org/10.1002/prot.22175
- Primary Citation of Related Structures:
2GKO, 2IXT, 3D43 - PubMed Abstract:
We determine and compare the crystal structure of two proteases belonging to the subtilisin superfamily: S41, a cold-adapted serine protease produced by Antarctic bacilli, at 1.4 A resolution and Sph, a mesophilic serine protease produced by Bacillus sphaericus, at 0.8 A resolution. The purpose of this comparison was to find out whether multiple calcium ion binding is a molecular factor responsible for the adaptation of S41 to extreme low temperatures. We find that these two subtilisins have the same subtilisin fold with a root mean square between the two structures of 0.54 A. The final models for S41 and Sph include a calcium-loaded state of five ions bound to each of these two subtilisin molecules. None of these calcium-binding sites correlate with the high affinity known binding site (site A) found for other subtilisins. Structural analysis of the five calcium-binding sites found in these two crystal structures indicate that three of the binding sites have two side chains of an acidic residue coordinating the calcium ion, whereas the other two binding sites have either a main-chain carbonyl, or only one acidic residue side chain coordinating the calcium ion. Thus, we conclude that three of the sites are of high affinity toward calcium ions, whereas the other two are of low affinity. Because Sph is a mesophilic subtilisin and S41 is a psychrophilic subtilisin, but both crystal structures were found to bind five calcium ions, we suggest that multiple calcium ion binding is not responsible for the adaptation of S41 to low temperatures.
Organizational Affiliation:
Department of Clinical Biochemistry, Faculty of Health Sciences, Ben-Gurion University, Beer Sheva 84105, Israel. almogo@bgu.ac.il