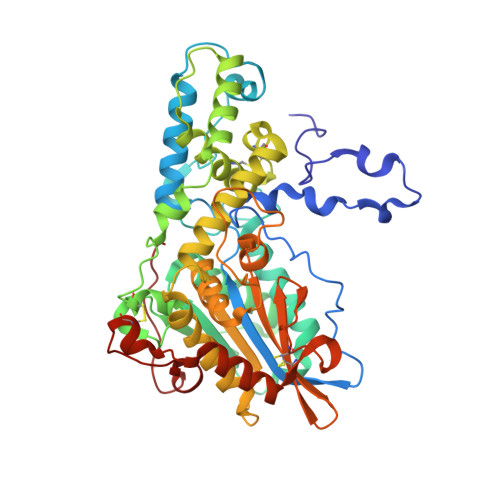
Structure of Debaryomyces castellii CBS 2923 phytase.
Ragon, M., Hoh, F., Aumelas, A., Chiche, L., Moulin, G., Boze, H.(2009) Acta Crystallogr Sect F Struct Biol Cryst Commun 65: 321-326
- PubMed: 19342770
- DOI: https://doi.org/10.1107/S1744309109008653
- Primary Citation of Related Structures:
2GFI - PubMed Abstract:
Phytate (myo-inositol hexakisphosphate) is the primary storage form of phosphate in seeds and legumes (Reddy et al., 1982). Phytases are phosphatases that hydrolyze phytate to less phosphorylated myo-inositol derivatives and inorganic phosphate. The crystal structure of phytase from Debaryomyces castellii has been determined at 2.3 A resolution. The crystals belonged to space group P6(5)22, with unit-cell parameters a = 121.65, c = 332.24 A. The structure was solved by molecular replacement and refined to a final R factor of 15.7% (R(free) = 20.9%). The final model consists of a dimer (with two monomers of 458 residues), five NAG molecules and 628 water molecules.
Organizational Affiliation:
UMR IR2B, Equipe Génie Microbiologique et Enzymatique, ENSAM-INRA, Montpellier, France.