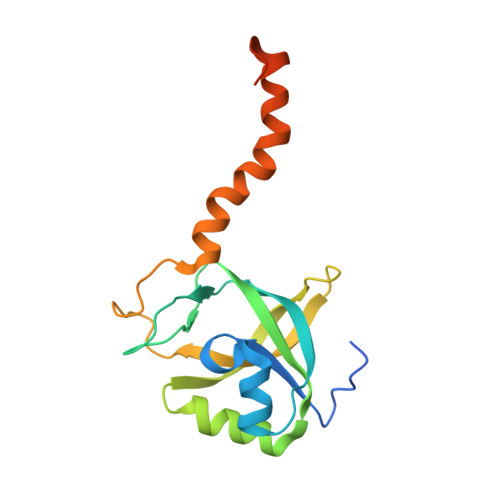
Solution structure of Arabidopsis thaliana protein At5g39720.1, a member of the AIG2-like protein family.
Lytle, B.L., Peterson, F.C., Tyler, E.M., Newman, C.L., Vinarov, D.A., Markley, J.L., Volkman, B.F.(2006) Acta Crystallogr Sect F Struct Biol Cryst Commun 62: 490-493
- PubMed: 16754964
- DOI: https://doi.org/10.1107/S1744309106015946
- Primary Citation of Related Structures:
2G0Q - PubMed Abstract:
The three-dimensional structure of Arabidopsis thaliana protein At5g39720.1 was determined by NMR spectroscopy. It is the first representative structure of Pfam family PF06094, which contains protein sequences similar to that of AIG2, an A. thaliana protein of unknown function induced upon infection by the bacterial pathogen Pseudomonas syringae. The At5g39720.1 structure consists of a five-stranded beta-barrel surrounded by two alpha-helices and a small beta-sheet. A long flexible alpha-helix protrudes from the structure at the C-terminal end. A structural homology search revealed similarity to three members of Pfam family UPF0131. Conservation of residues in a hydrophilic cavity able to bind small ligands in UPF0131 proteins suggests that this may also serve as an active site in AIG2-like proteins.
Organizational Affiliation:
Center for Eukaryotic Structural Genomics, USA.