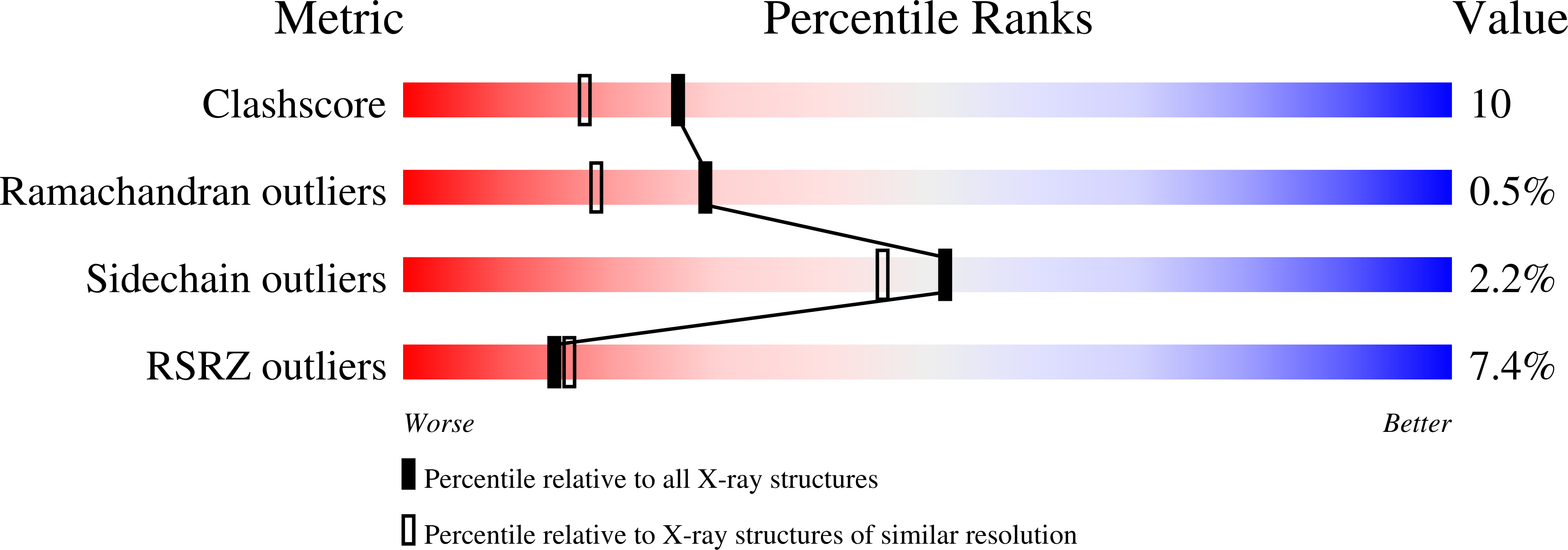The Extraordinary Specificity of Xanthine Phosphoribosyltransferase from Bacillus subtilis Elucidated by Reaction Kinetics, Ligand Binding, and Crystallography
Arent, S., Kadziola, A., Larsen, S., Neuhard, J., Jensen, K.F.(2006) Biochemistry 45: 6615-6627
- PubMed: 16716072
- DOI: https://doi.org/10.1021/bi060287y
- Primary Citation of Related Structures:
2FXV - PubMed Abstract:
Xanthine phosphoribosyltransferase (XPRTase) from Bacillus subtilis is a representative of the highly xanthine specific XPRTases found in Gram-positive bacteria. These XPRTases constitute a distinct subclass of 6-oxopurine PRTases, which deviate strongly from the major class of H(X)GPRTases with respect to sequence, PRPP binding motif, and oligomeric structure. They are more related with the PurR repressor of Gram-positive bacteria, the adenine PRTase, and orotate PRTase. The catalytic function and high specificity for xanthine of B. subtilis XPRTase were investigated by ligand binding studies and reaction kinetics as a function of pH with xanthine, hypoxanthine, and guanine as substrates. The crystal structure of the dimeric XPRTase-GMP complex was determined to 2.05 A resolution. In a sequential reaction mechanism XPRTase binds first PRPP, stabilizing its active dimeric form, and subsequently xanthine. The XPRTase is able also to react with guanine and hypoxanthine albeit at much lower (10(-)(4)-fold) catalytic efficiency. Different pK(a) values for the bases and variations in their electrostatic potential can account for these catalytic differences. The unique base specificity of XPRTase has been related to a few key residues in the active site. Asn27 can in different orientations form hydrogen bonds to an amino group or an oxo group at the 2-position of the purine base, and Lys156 is positioned to make a hydrogen bond with N7. This and the absence of a catalytic carboxylate group near the N7-position require the purine base to dissociate a proton spontaneously in order to undergo catalysis.
Organizational Affiliation:
Centre for Crystallographic Studies, Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø, Denmark.