Structure and function of the 3-carboxy-cis,cis-muconate lactonizing enzyme from the protocatechuate degradative pathway of Agrobacterium radiobacter S2.
Halak, S., Lehtio, L., Basta, T., Burger, S., Contzen, M., Stolz, A., Goldman, A.(2006) FEBS J 273: 5169-5182
- PubMed: 17054713
- DOI: https://doi.org/10.1111/j.1742-4658.2006.05512.x
- Primary Citation of Related Structures:
2FEL, 2FEN - PubMed Abstract:
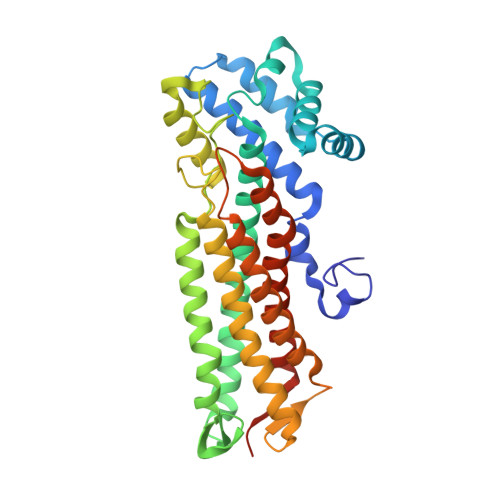
3-carboxy-cis,cis-muconate lactonizing enzymes participate in the protocatechuate branch of the 3-oxoadipate pathway of various aerobic bacteria. The gene encoding a 3-carboxy-cis,cis-muconate lactonizing enzyme (pcaB1S2) was cloned from a gene cluster involved in protocatechuate degradation by Agrobacterium radiobacter strain S2. This gene encoded for a 3-carboxy-cis,cis-muconate lactonizing enzyme of 353 amino acids - significantly smaller than all previously studied 3-carboxy-cis,cis-muconate lactonizing enzymes. This enzyme, ArCMLE1, was produced in Escherichia coli and shown to convert not only 3-carboxy-cis,cis-muconate but also 3-sulfomuconate. ArCMLE1 was purified as a His-tagged enzyme variant, and the basic catalytic constants for the conversion of 3-carboxy-cis,cis-muconate and 3-sulfomuconate were determined. In contrast, Agrobacterium tumefaciens 3-carboxy-cis,cis-muconate lactonizing enzyme 1 could not, despite 87% sequence identity to ArCMLE1, use 3-sulfomuconate as substrate. The crystal structure of ArCMLE1 was determined at 2.2 A resolution. Consistent with the sequence, it showed that the C-terminal domain, present in all other members of the fumarase II family, is missing in ArCMLE1. Nonetheless, both the tertiary and quaternary structures, and the structure of the active site, are similar to those of Pseudomonas putida 3-carboxy-cis,cis-muconate lactonizing enzyme. One principal difference is that ArCMLE1 contains an Arg, as opposed to a Trp, in the active site. This indicates that activation of the carboxylic nucleophile by a hydrophobic environment is not required for lactonization, unlike earlier proposals [Yang J, Wang Y, Woolridge EM, Arora V, Petsko GA, Kozarich JW & Ringe D (2004) Biochemistry43, 10424-10434]. We identified citrate and isocitrate as noncompetitive inhibitors of ArCMLE1, and found a potential binding pocket for them on the enzyme outside the active site.
Organizational Affiliation:
Institut für Mikrobiologie, Universität Stuttgart, Germany.