Biochemical Characterization and Crystal Structure of Synechocystis Arogenate Dehydrogenase Provide Insights into Catalytic Reaction
Legrand, P., Dumas, R., Seux, M., Rippert, P., Ravelli, R., Ferrer, J.-L., Matringe, M.(2006) Structure 14: 767-776
- PubMed: 16615917
- DOI: https://doi.org/10.1016/j.str.2006.01.006
- Primary Citation of Related Structures:
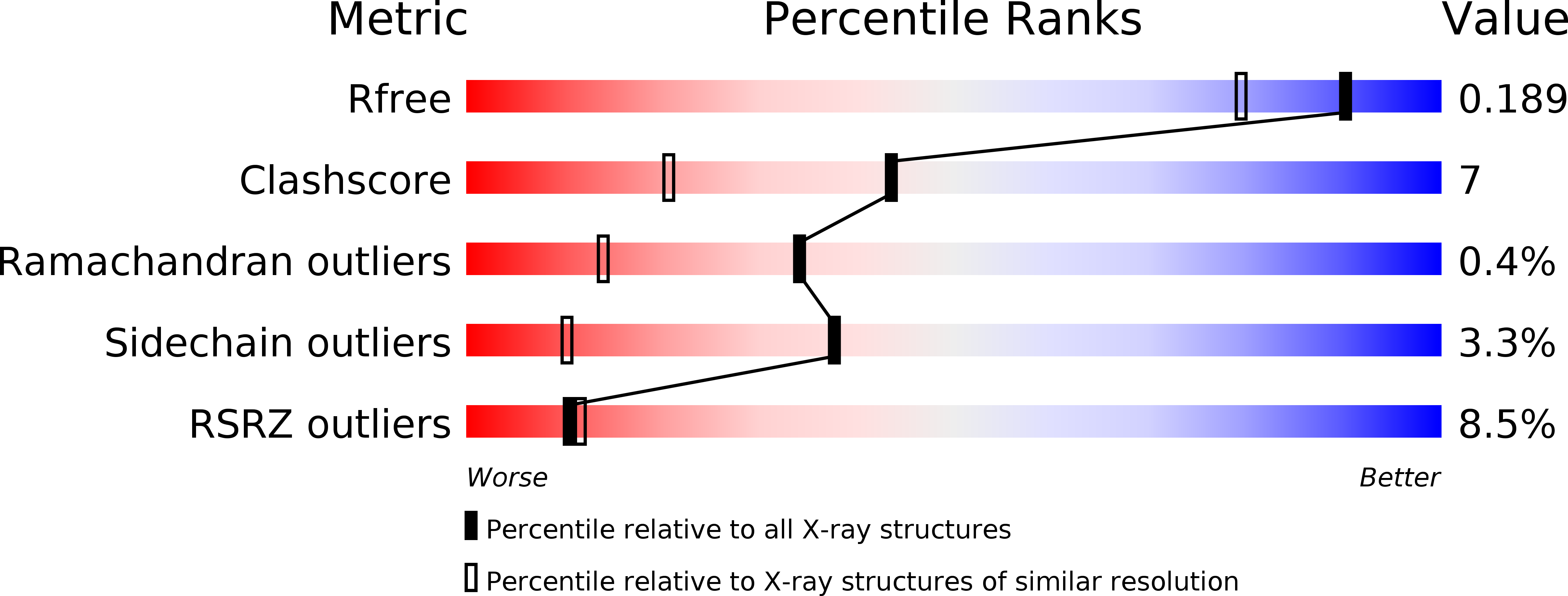
2F1K - PubMed Abstract:
The extreme diversity in substrate specificity, and in the regulation mechanism of arogenate/prephenate dehydrogenase enzymes in nature, makes a comparative structural study of these enzymes of great interest. We report here on the biochemical and structural characterization of arogenate dehydrogenase from Synechocystis sp. (TyrAsy). This work paves the way for the understanding of the structural determinants leading to diversity in substrate specificity, and of the regulation mechanisms of arogenate/prephenate dehydrogenases. The overall structure of TyrAsy in complex with NADP was refined to 1.6 A. The asymmetric unit contains two TyrAsy homodimers, with each monomer consisting of a nucleotide binding N-terminal domain and a particularly unique alpha-helical C-terminal dimerization domain. The substrate arogenate was modeled into the active site. The model of the ternary complex enzyme-NADP-arogenate nicely reveals at the atomic level the concerted mechanism of the arogenate/prephenate dehydrogenase reaction.
Organizational Affiliation:
Institut de Biologie Structurale Jean-Pierre Ebel, Centre National de la Recherche Scientifique, Université Joseph Fourier, Laboratoire de Cristallographie et Cristallogenèse des Protéines/Groupe Synchrotron, 38027 Grenoble cedex 1, France.