Structural basis for diacetylated histone H4 tail recognition by the second bromodomain of human BRD2
Padmanabhan, B., Umehara, T., Nakano, K., Jang, M.K., Ozato, K., Yokohama, S.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Bromodomain-containing protein 2 | 112 | Homo sapiens | Mutation(s): 0 Gene Names: BRD2, KIAA9001, RING3 | 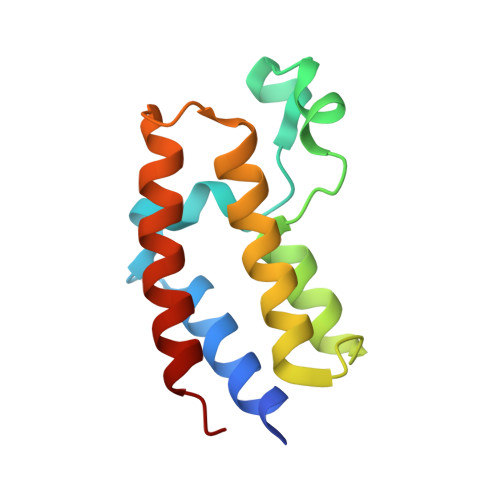 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P25440 (Homo sapiens) Explore P25440 Go to UniProtKB: P25440 | |||||
PHAROS: P25440 GTEx: ENSG00000204256 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P25440 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 15-mer peptide from Histone H4 | E [auth Q], F [auth R] | 15 | N/A | Mutation(s): 0 | 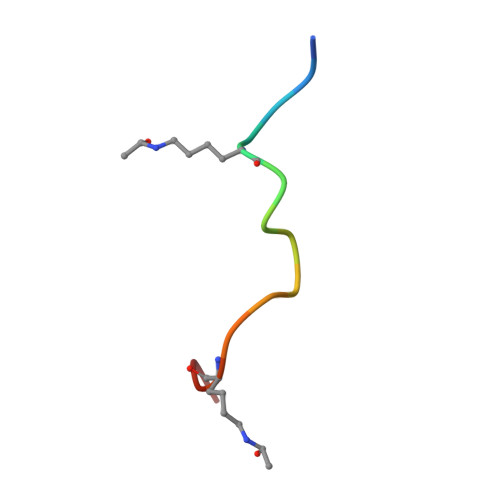 |
UniProt | |||||
Find proteins for P02309 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P02309 Go to UniProtKB: P02309 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P02309 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| ALY Query on ALY | E [auth Q], F [auth R] | L-PEPTIDE LINKING | C8 H16 N2 O3 |  | LYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 45.828 | α = 90 |
| b = 128.591 | β = 104.7 |
| c = 45.847 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| ADSC | data collection |
| HKL-2000 | data reduction |
| SCALEPACK | data scaling |
| MOLREP | phasing |